
Previous PDUFA Performance Reports identified several important outcomes that had resulted from the Agency's meeting and exceeding its application review performance commitments. These included increasing numbers of applications filed, higher quality applications, and quicker approvals for products with the requisite data, outcomes that result in more quality products reaching American practitioners and consumers faster. In FY 2000 the Agency continued to exceed nearly all the review performance goals of PDUFA II1 despite the goals becoming more challenging each year. Application filings and quality remain high by historic standards, and approval times continue to drop.
 [d]
[d]
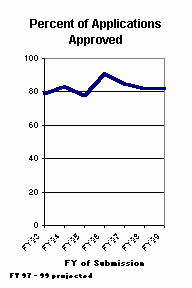
The percentage of filed new product applications that ultimately are approved increased from approximately 66 percent in the pre-PDUFA years2 to roughly 80 percent for applications submitted from FY 93 through FY 95. These early PDUFA cohorts are essentially finished; only one submission from earlier than FY 96 was approved in FY 00. Approval rates currently stand at 77 percent for FY 97 applications, 69 percent for FY 98 applications, and 56 percent for FY 99 applications. The final approval rates for all of these years should be above 80 percent if present trends hold.
Compared to the approval rates for all new drug applications, there is a smaller increase in approval rates for new molecular entities (NMEs), unique new drugs that are approved for the first time by FDA. In the years before PDUFA (FY 88 to FY 92), 76 percent of the NMEs were ultimately approved. Since PDUFA, that rate has risen to approximately 81 percent.
 [d]
[d]
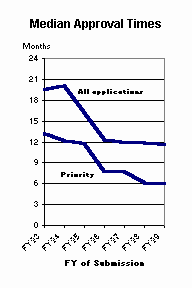
The median total approval time for new product applications submitted in FY 99 dropped to 11.6 months3. Total approval time is the time from the initial submission of a marketing application to the issuance of an approval letter for that application. It includes both FDA's review time and the time the sponsor spends answering deficiencies noted by FDA and can encompass several review 'cycles.' Given the progression of PDUFA II review goals, median approval times may drop to 10 months in FY 01 or FY 02 if the current rate of first review cycle approvals is sustained.
Median total approval times for priority applications submitted in FY 99 was 6 months3, less than half the median approval times for priority applications submitted in the early PDUFA years. The products of priority applications represent significant therapeutic gains and are an important outcome for the consumer and the medical community.
 [d]
[d]
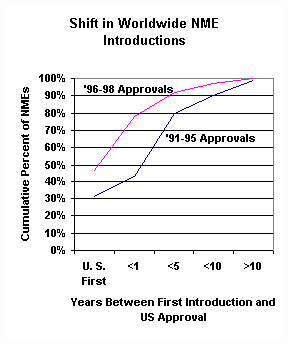
In the years since the passage of PDUFA, there has been a shift in the pattern of NME introductions in the world. According to information from the Tufts University Center for the Study of Drug Development presented at the PDUFA public meeting on September 15, 2000, only 43 percent of the NMEs approved in the U. S. in 1991-1995, primarily pre-PDUFA submissions, received that approval within a year of its first introduction on the world market.
That percentage almost doubled to 80 percent for the NMEs approved in the U.S. from 1996-1998, primarily post-PDUFA submissions. Increasingly, American patients are receiving the benefits of important new drugs before they are available to citizens of other countries.
This important benefit, however, brings with it a need for increased surveillance. Although the Agency's high standards for safety and efficacy have not changed under PDUFA, the pre-market approval process cannot detect all possible future safety issues. Once a new drug is approved, safety issues sometimes arise simply because of its much wider use. Historically, about 3 to 4 percent of the NMEs approved in the world have eventually been withdrawn from the market for safety reasons. Because more of these products are now marketed in the U.S. first, FDA recognizes that it must be increasingly vigilant in its post-market surveillance efforts.
Report on PDUFA Goals | Table of Contents | Previous Page
FDA Home Page | Search | A-Z Index | Site Map | Contact FDA
FDA/Office of Planning
Web page created by cm 2001-MAR-01.