


Last year's PDUFA Performance Report, which marked the end of the original Prescription Drug User Fee Act (PDUFA I 1 ), reported on several important outcomes that had resulted from the Agency's meeting and exceeding its performance commitments. These included more applications filed, better applications, and quicker approvals; outcomes that result in more products reaching American consumers faster. This year, while the Agency's PDUFA II1 goals continue their annual progression toward higher performance levels, some of the outcome measures appear to have approached their limits, and additional future gains may be small.
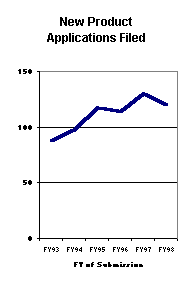
Fewer Applications Filed: Since the start of PDUFA I, annual submissions of new product applications have increased from 88 to 120, an average increase of more than 6 percent annually. Efficacy supplement receipts also increased an average of 6 percent per year, and manufacturing supplements increased 8 percent per year. FY 98 submissions, however, ran counter to this long term trend; new product submissions dropped from 130 to 120 and efficacy supplements dropped from 162 to 132. Only manufacturing supplement receipts continued to grow, increasing from 1,600 to 1,830.
More Priority Applications: The drop in new product applications was offset somewhat by an increase in the number of priority applications. Priority applications accounted for between 20% and 25% of all applications filed each year from FY 93 through FY 97; in FY 98 they jumped to 32%. The products of priority applications represent significant therapeutic gains and are an important outcome for the consumer and the medical community.
High and Stable Approval Rates: The percentage of filed applications that ultimately are approved has increased from the less than 60 percent rate of the pre-PDUFA years2 and now appears to have stabilized. Between 80 and 83 percent of the applications submitted from FY 93 through FY 96 have been approved. The early PDUFA cohorts are almost finished; only 3 submissions from FY 93 and 1 from FY 94 were approved in FY 98. For the later PDUFA years, FY 95 - FY 97, it appears that final approval rates will be about 85 percent.
More than half of all the approval decisions are now made on the initial review cycle. This increase from the early PDUFA years, when only about 25 percent of the approvals came on the first review cycle, suggests that submission quality has improved significantly. Besides contributing the shortened approval times, higher initial approval rates mean fewer resubmissions. In FY 96, FDA received 103 resubmissions; there were only 73 in FY 98.
Quick and Steady Approval Times: The total approval times for applications submitted during the PDUFA years has leveled at a 12 month median3. If 85 percent of the FY 97 submissions are approved, the median approval time will be 12 months. This is the same as the median approval time for the FY 96 submissions and is an improvement over the 16.3 month median of the FY 95 submissions, the 19 to 20 month medians of the FY 93 and FY 94 submissions, and the 23 month median typical of the early 1990s. Given the progression of PDUFA II review performance goals, median approval times will likely drop to 10 months in FY 2001 or FY 2002 if the current rate of first review approvals is sustained.