 |

Melanoma Incidence Among Young Women in the U.S. is Rising
The annual incidence of invasive cutaneous melanoma, the deadliest form of skin cancer, increased among Caucasian women in the United States aged 15 to 39 by 50 percent between 1980 and 2004, investigators from NCI's Division of Cancer Epidemiology and Genetics reported online July 10 in the Journal of Investigative Dermatology. The incidence among Caucasian men in the United States did not increase significantly over the same time period.
"We have known for some time that melanoma incidence has been consistently increasing among older adults in the United States," says Dr. Mark Purdue, lead author of the study. "What has not been clear is whether the melanoma trends among younger adults have been changing. Some studies published in the 1990s had suggested that melanoma rates were leveling off in this age group. However, a study conducted by our group in 2001 saw evidence that melanoma incidence was still increasing among young women. Our present study, which includes an additional 7 years of data, was conducted to clarify what trends are taking place among young adults."
The investigators used nine NCI Surveillance, Epidemiology, and End Results registries that had collected incidence and mortality data since 1973. They found that the rate of increase in incidence for young women declined from 1978 to 1987 and then stabilized until 1992, but began rising again afterward. In absolute numbers, the annual incidence increased from 5.5 cases per 100,000 persons in 1973 to 13.9 per 100,000 in 2004.
Read more 


Nanoparticles Deliver Chemotherapy and Block Cancer's Spread
By using targeted nanoparticles carrying significantly reduced doses of chemotherapy, researchers have demonstrated the ability to preferentially block the spread of cancer, while largely sparing the surrounding tissues. A series of experiments in animals with forms of pancreatic and kidney cancer showed that the nanotechnology therapy consistently reduced the incidence of metastasis by 90 percent as compared with untreated mice.
The results, reported online July 8 in the Proceedings of the National Academy of Sciences, suggest possible new approaches for inhibiting tumor angiogenesis, the formation of blood vessels that supply tumors with the nutrients needed to grow and spread. Dr. David Cheresh, a participant in the NCI Center of Cancer Nanotechnology Excellence (CCNE) at the University of California, San Diego (UCSD), led the study. Dr. Cheresh leads efforts to develop "smart" nanoparticle platforms at
the Center.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Melanoma Incidence Among Young Women in the U.S. is Rising
The annual incidence of invasive cutaneous melanoma, the deadliest form of skin cancer, increased among Caucasian women in the United States aged 15 to 39 by 50 percent between 1980 and 2004, investigators from NCI's Division of Cancer Epidemiology and Genetics reported online July 10 in the Journal of Investigative Dermatology. The incidence among Caucasian men in the United States did not increase significantly over the same time period.
"We have known for some time that melanoma incidence has been consistently increasing among older adults in the United States," says Dr. Mark Purdue, lead author of the study. "What has not been clear is whether the melanoma trends among younger adults have been changing. Some studies published in the 1990s had suggested that melanoma rates were leveling off in this age group. However, a study conducted by our group in 2001 saw evidence that melanoma incidence was still increasing among young women. Our present study, which includes an additional 7 years of data, was conducted to clarify what trends are taking place among young adults."
The investigators used nine NCI Surveillance, Epidemiology, and End Results registries that had collected incidence and mortality data since 1973. They found that the rate of increase in incidence for young women declined from 1978 to 1987 and then stabilized until 1992, but began rising again afterward. In absolute numbers, the annual incidence increased from 5.5 cases per 100,000 persons in 1973 to 13.9 per 100,000 in 2004.
The increase in incidence among young women was not limited to early, thin lesions; increasing trends were also observed for thicker and advanced-stage (both regional and metastatic) melanomas. If the investigators had observed an increase only in thin lesions, this could have indicated that the incidence findings were due to increased detection of early stage disease because of improved melanoma awareness and surveillance since the early 1980s, explains Dr. Purdue. But the fact that incidence of later-stage disease also increased suggests that the observed rise in incidence is real.
When they compared the data by birth cohorts (people grouped into 5-year periods of birth), the investigators found that melanoma incidence increased for women born after 1965. "The observed increase in incidence among women born after 1965 is consistent with a birth cohort effect," conclude the authors, meaning that the increase indicates a change in exposure to risk factors for disease across cohorts of people born in different years.
"We can't tell from this data what exactly caused this increase in incidence among young women, but one possible explanation is that an increase in UV exposure, a risk factor for melanoma, may be responsible," concludes Dr. Purdue.
Previous research has shown that the prevalence of sunburn is increasing for adults in the U.S., as is tanning bed usage, particularly among young women. "Additional studies are needed to clarify whether the increasing trends for melanoma…are the result of changes in [ultraviolet radiation] exposure in the population," state the papers' authors.
People concerned about ultraviolet radiation can reduce their exposure by staying out of the sun when its rays are the strongest (between 10 am and 4 pm), wearing a broad-brimmed hat and protective clothes when outside, using sunscreen with a sun protective factor (SPF) of 15 or higher, and not seeking a tan.
—Sharon Reynolds
|
 |
 |
Nanoparticles Deliver Chemotherapy and Block Cancer's Spread
By using targeted nanoparticles carrying significantly reduced doses of chemotherapy, researchers have demonstrated the ability to preferentially block the spread of cancer, while largely sparing the surrounding tissues. A series of experiments in animals with forms of pancreatic and kidney cancer showed that the nanotechnology therapy consistently reduced the incidence of metastasis by 90 percent as compared with untreated mice.
The results, reported online July 8 in the Proceedings of the National Academy of Sciences, suggest possible new approaches for inhibiting tumor angiogenesis, the formation of blood vessels that supply tumors with the nutrients needed to grow and spread. Dr. David Cheresh, a participant in the NCI Center of Cancer Nanotechnology Excellence (CCNE) at the University of California, San Diego (UCSD), led the study. Dr. Cheresh leads efforts to develop "smart" nanoparticle platforms at
the Center.
"Particularly significant and promising is the fact that tumor metastases were reduced in the treated animals," said Dr. Piotr Grodzinski, program director for the NCI Alliance for Nanotechnology in Cancer.
The research builds upon a discovery made earlier by Dr. Cheresh's group showing that a protein called integrin ανβ3 is often found on growing tumor blood vessels but not on preexisting ones. The lipid-based nanoparticles were designed to attach to the protein receptor and deliver doxorubicin, a chemotherapeutic agent typically delivered systemically but with undesirable toxic side-effects.
By encapsulating and targeting the drug through the use of these nanoparticles, the investigators observed a 15-fold improvement in drug efficacy relative to conventional delivery. While the effect on primary tumors was modest, treatment with the nanoparticles was effective in preventing dissemination of the cancer. The researchers noted that this is a highly significant finding since more than 90 percent of patients with solid tumors die due to metastasis.
Dr. Cheresh noted that the CCNE researchers are continuing their work with collaborators at the Moores UCSD Cancer Center to repeat the doxorubicin experiments with newer agents that target additional pathways involved in angiogenesis.
Fewer Americans Exposed to Secondhand Smoke
The prevalence of self-reported secondhand smoke (SHS) exposure at home and changes in any exposure, as measured by serum cotinine (a biologic indicator of recent SHS exposure), declined significantly in nonsmoking children, adolescents, and adults in recent years, according to a report in the July 11 Morbidity and Mortality Weekly Report (MMWR).
The MMWR analysis used data from the National Health and Nutrition Examination Surveys to determine at-home SHS exposure and serum cotinine levels in nonsmokers aged 4 years and older, from 1988-1994 and from 1999-2004. The percentage of U.S. nonsmokers who reported SHS exposure at home declined from 20.9 percent to 10.2 percent between the two time periods. Similarly, the percent of nonsmokers with any exposure to SHS declined significantly, from 83.9 percent to 46.4 percent.
However, between 1999 and 2004, children and adolescents remained at higher risk of SHS exposure than adults, with nearly one in four children aged 4 to 11 and one in five adolescents aged 12 to 19 exposed to SHS in the home, compared with only one in twenty adults aged 20 years or older. Non-Hispanic blacks and low-income Americans were
also at significantly higher risk of
SHS exposure.
The authors attribute the broad declines in SHS exposure to laws and policies that restrict or eliminate smoking in workplaces and public places, the increased percentage of households that have rules against smoking in the home, and the declining prevalence of smoking among Americans. The authors note that "the results of this study underscore the need for ongoing prevention efforts to reduce SHS exposure with strategies that focus on protection for those at greatest risk."
Gene Signatures Point to Therapy for Neuroblastoma
Patients with neuroblastoma may benefit from the combination of a histone deacetylase (HDAC) inhibitor and a retinoid, two types of drugs used in differentiation therapy (an approach which causes cancerous cells to resume the process of maturation and differentiation into mature cells), researchers report. Neuroblastoma is a cancer of the nervous system in children, and differentiation therapy is a potential strategy for treating the disease.
Dr. Kimberly Stegmaier of Dana-Farber Cancer Institute and her colleagues used a high-throughput screening strategy to identify drugs that could be used in combination with HDAC inhibitors to cause neuroblastoma cells to differentiate. They screened more than a thousand compounds to find those that could induce a genetic signature associated with differentiation in neuroblastoma cells.
The best candidate was all-trans retinoic acid, a type of retinoid. Further studies showed that the combination of an HDAC inhibitor and a retinoid resulted in greater neuroblastoma differentiation than either alone, while there was also evidence of synergistic effects on cell death. The combination therapy led to the longest survival among mice with a form of neuroblastoma.
The findings, reported online July 8 in Proceedings of the National Academy of Sciences, coincide with a recent phase I study showing that this type of drug combination was well tolerated in children with a variety of cancers, including neuroblastoma. The drugs have also been tested extensively as single agents in adults.
"One of the most exciting aspects of this work was that as the study neared completion, we learned that the drug combination was well tolerated in children," said Dr. Stegmaier,
a pediatric oncologist. "Our study adds to the growing body of data
that this combination of drugs
may make sense for patients with neuroblastoma."
Study Suggests Therapeutic Avenue for Transplant-Related Cancers
Results from a new study suggest that certain anti-angiogenic agents could be used to prevent or treat the cancers that occur in 15-20 percent of patients who receive an organ transplant. The study, which relied on laboratory and animal models, showed that a common and effective immunosuppressive agent used in organ transplant procedures increases the expression of vascular endothelial growth factor (VEGF), which is critical to a tumor's ability to develop blood vessels and recruit a blood supply. The study was published July 15 in Cancer Research.
"It may be that anti-VEGF agents given judiciously after transplantation can reduce future cancer occurrence," said the study's lead author, Dr. Soumitro Pal of the Transplantation Research Center at Children's Hospital Boston and Brigham and Women's Hospital. "We would need to figure out how to balance benefit and risk to keep cancer at bay."
Although there are several potential causes of cancer in patients following a transplant, the authors noted, this study focused on whether the drug cyclosporine could promote the growth of pre-existing "microtumors" in a mouse model of post-transplantation kidney cancer. First, however, they used kidney cancer cell lines to demonstrate that cyclosporine caused activation of the VEGF gene, that the extent of this activation was dose dependent, and that it increased expression of the VEGF protein. They found that cyclosporine also activates the intracellular signaling pathway PKC, which in turn increases the transcription of the VEGF gene.
Tumor growth was greater in mice given cyclosporine than untreated mice, they discovered. But simultaneously treating the mice with VEGF inhibitors slowed tumor growth. The authors noted that other studies have found pathways other than PKC via which cyclosporine could increase VEGF expression.
|
 |
 |

Developing a Clinical Trials System for 21st Century Science
One of the resounding successes of cancer research over the last several decades has been the productivity of our clinical trials system. NCI-supported clinical trials have brought important new interventions to patients, including a cervical cancer vaccine, new targeted therapies for treating advanced colorectal and kidney cancer, and adjuvant therapies for the treatment of breast cancer, to name just a few.
Clinical trials are and will continue to be the best means for proving that a given treatment can effectively and safely treat cancer. But as was reported last week in the NCI Cancer Bulletin, there is a consensus that the current system for conducting clinical trials, while it has served the community and patients admirably, is no longer a good fit for 21st-century biomedical cancer research.
As I told participants at the Institute of Medicine-sponsored National Cancer Policy Forum (NCPF) on phase III cancer clinical trials and NCI's cooperative group program, the current system has become inefficient, with insufficient funding, duplicative efforts, and a regulatory climate that has made it difficult to swiftly answer important clinical questions. In addition, the very nature of biomedical science is rapidly changing. Our reliance on nonspecific, broadly toxic therapies is quickly being supplanted by the emergence of newer, more targeted therapies that, particularly when used in combination, hold the promise of increased efficacy with less - or more manageable - toxicities.
In response, we must look beyond the horizon in planning how future trials will need to be designed, managed, and funded.
Some of this work is already underway in the form of changes being made in response to the recommendations of the Clinical Trials Working Group and
Translational Research Working Group
. Those initiatives, however, are limited in scope and, by their very nature, cannot address some of the broader, systemic
challenges of conducting phase III clinical trials.
There are a number of potential changes to the system that I believe can begin to refashion the clinical
trials system for the era of personalized oncology.
Among these is the development of incentives geared toward improving participation in clinical trials. Such incentives must include working with third-party payers to improve reimbursement for coverage of trial participants' care, as well as other incentives to reward high-accruing sites and aid the professional advancement of clinicians who lead or participate in clinical trials.
It is also likely that there will be a gradual shifting of resources to support the development of a true, linked clinical trials network. Such a network will utilize programs like the cancer Biomedical Informatics Grid to share information and resources, and will have dedicated tumor characterization centers. These centers will be vital if we are to transform our clinical studies toward those that test interventions based upon extensive characterization of individual patients' tumors and the surrounding microenvironment.
The increased use of the NCI-supported centralized Institutional Review Board (IRB) also is of critical importance. This was a topic of significant debate during the NCPF meeting. Clearly there are matters to be ironed out, and NCI can play a role in helping to address governance concerns with regard to local IRBs versus the central IRB, and to better promote the advantages of using a central IRB in developing and launching clinical trials.
This is by no means an exhaustive list of changes or remedies that need to be made. Next year recommendations will be forthcoming from the IOM, based on this recent meeting, and those will be fully considered.
In the meantime, NCI will continue its dialogue with clinical researchers, the leaders of the cooperative groups, the advocacy community, and the Food and Drug Administration and other federal agencies whose regulations and policies affect how clinical trials are designed and run. The entire cancer community has a role to play in improving the clinical trials system. The goal is a top priority,
and this work will influence the conduct of cancer research for decades to come.
Dr. John E. Niederhuber
Director, National Cancer Institute
|
 |
 |

The New Face of Head and Neck Cancer Treatment
Rarely do cancer clinical trials have a poster patient. But a current randomized phase II trial led by researchers from the University of Chicago has just that. Grant Achatz has been the subject of feature stories in magazines like The New Yorker and Chicago, partly because he's a highly celebrated young chef, but also because he had a malignant tumor that, prior to treatment, had engulfed most of his tongue.
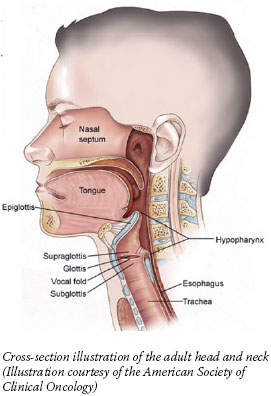 Mr. Achatz's case cast a spotlight on head and neck cancer, which includes cancers of the mouth, nasal cavity, and throat. It has also highlighted the significant progress made in treating patients with large, localized tumors but no apparent metastases (which represents three-quarters of head and neck cancer patients), including the option in many cases to avoid disfiguring surgery and save vital organs without risking survival.
Mr. Achatz's case cast a spotlight on head and neck cancer, which includes cancers of the mouth, nasal cavity, and throat. It has also highlighted the significant progress made in treating patients with large, localized tumors but no apparent metastases (which represents three-quarters of head and neck cancer patients), including the option in many cases to avoid disfiguring surgery and save vital organs without risking survival.
Much of that progress is a result of the innovative use of two traditional treatments, chemotherapy and radiation. And new research is pointing to more treatment options on the horizon, including molecularly targeted agents and potential markers that may define who the best candidates are for a given treatment.
No surgery needed
Oncologists at three leading head and neck programs saw Mr. Achatz and recommended a glossectomy, removal of most of his tongue. He chose, however, to follow a fourth opinion, enrolling in a clinical trial testing an "organ preservation" regimen. And according to media reports, he is currently disease-free. The fact that there was an option that allowed him to avoid surgery and keep his tongue shows just how far treatment for these cancer types has come.
"It's a very exciting time in head and neck cancer research," says Dr. Arlene Forastiere from the Johns Hopkins School of Medicine, who led the clinical trial that established the concurrent use of chemotherapy and radiation to successfully treat cancers of the larynx in place of surgical removal. Similar results have been seen for cancers of the oropharynx, which includes the tonsils and base of the tongue.
"We've achieved a lot with current therapies that are cisplatin-based chemoradiation," she says. "But we certainly still have room to improve in terms of cure rates," she acknowledges. Five-year survival rates for oropharynx cancers, for example, hover around 59 percent.
Mr. Achatz's case, explains Dr. Everett Vokes, who is leading the trial in which Mr. Achatz is enrolled, is fairly rare. The tumor began on the side of the tongue - often called the "oral tongue," and not considered part of the oropharynx - whereas most tongue cancers begin at the base. It also took several years to develop, while most cancers in the oral cavity are thought to progress rapidly. Mr. Achatz did not smoke and drank alcohol only moderately, eliminating two significant causative factors in such a young patient.
For small tongue lesions, Dr. Vokes explains, surgery is typically the best option. And for a case like Mr. Achatz's, for which there is little published data, most head and neck cancer programs would recommend surgery. "And we said, 'Maybe he should…but let's try concurrent chemoradiation first,'" Dr. Vokes recalls.
Beyond concurrent chemoradiation, the trial in which Mr. Achatz is enrolled compares the addition of cetuximab (Erbitux) - a monoclonal antibody that targets the epidermal growth factor receptor (EGFR), which is often overexpressed in
head and neck cancers - to either of two different chemoradiation regimens. Trial participants also receive induction chemotherapy, which is given before "definitive" therapy to prevent metastases.
Based on clinical trial findings published in 2006, cetuximab is already approved for the treatment of head and neck cancer, in combination with radiation therapy. But, explains Dr. Francis Worden, of the University of Michigan Comprehensive Cancer Center, its use at this point is limited to patients who, for various reasons, would not be candidates for the toxic platinum-based chemotherapy used in standard chemoradiation.
In addition to helping better define the role of EGFR-targeted agents, the phase II trial in which Mr. Achatz is enrolled, as well as four large phase III trials underway, will provide further evidence about the value of induction chemotherapy, which, while extensively studied, is not yet a standard of care.
Even concurrent chemoradiation - which comes at the cost of severe toxicities that can significantly inhibit a patient's ability to swallow, talk, and taste for a period of time - is under investigational scrutiny. Dr. Vokes and colleagues, for example, just published the results of a phase II clinical trial showing that the radiation dose can be gradually lowered throughout treatment without sacrificing efficacy.
A Surprising "Benefit" of
HPV Infection?
Head and neck cancer rates had been declining thanks to lower smoking rates. However, that downturn has stagnated thanks to a rise in oropharynx cancers, mostly among people aged 45 or younger, that has been traced to human papillomavirus (HPV) infections, namely infection with HPV 16.
If there is a positive note to be
taken from this concerning trend, researchers say, it's that a number
of observational studies have
shown that HPV-positive patients have better outcomes than HPV-negative patients.
"We know now that these are two different tumors," says Dr. Worden, whose group just published results from a small trial in which nearly four out of five HPV-positive patients with advanced oropharynx cancer were alive 4 years after starting treatment, compared with approximately one in four HPV-negative patients. The available data, he adds, suggest that HPV-positive patients could be treated with a less toxic regimen.
That's a theory that's ready to be tested, says Dr. Forastiere. The NCI-sponsored Eastern Cooperative Oncology Group is launching clinical trials that will stratify participants by their HPV status, reserving a less demanding regimen of chemotherapy and radiation for HPV-positive patients, while HPV-negative
patients will be treated with standard chemoradiation and an EGFR-targeted agent.
—By Carmen Phillips
|
 |
 |

Childhood Cancer Bill Cleared for President's Signature
On July 16, the U.S. Senate voted to pass HR 1553, the Caroline Pryce Walker Conquer Childhood Cancer Act of 2008. HR 1553 was introduced in the U.S. House of Representatives on March 15, 2007 by Congresswoman Deborah Pryce (R-OH). A companion bill, S. 911, was introduced in the Senate by Congressman Jack Reed (D-RI)
4 days later.
The legislation authorizes the allocation of $30 million per year from fiscal year 2009 through 2013 to support pediatric cancer research, establish a childhood cancer database, and provide information about the diseases to affected families. Despite the authorization levels specified in the bill, HR 1553 does not appropriate the $30 million. Funding for the programs described in the bill must come from the existing budget or new appropriations.
The House Energy and Commerce Committee marked up the bill on May 7, at which time Congresswoman Hilda Solis (D-CA) proposed an amendment to ensure that the public awareness provisions in the bill would be culturally and linguistically appropriate for minority and medically underserved patients and families. This amendment also renamed the bill in honor of Representative Pryce's daughter, who died of cancer in 1999 at age 9.
The bill was overwhelmingly supported in the House and passed on June 12 by roll call vote, 416-0. The measure then passed the Senate by unanimous consent and will now be sent to the President for his signature.
|
 |
 |

Treating Relapsed or Refractory B-cell Lymphomas
Name of the Trial
Phase I/II Study of Flavopiridol in Patients with Refractory or Recurrent Mantle Cell Lymphoma or Diffuse Large B-Cell Lymphoma (NCI-07-C-0081). See the protocol summary at http://cancer.gov/clinicaltrials/NCI-07-C-0081.
Principal Investigator
Dr. Kieron Dunleavy, NCI Center for Cancer Research
Why This Trial Is Important
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL), accounting for up to 30 percent of new cases. DLBCL is an aggressive lymphoma, and, although many patients can be cured with current therapies, the prognosis for patients with relapsed DLBCL is often poor. Mantle cell lymphoma (MCL) is a less-common type of NHL; however, it is usually not curable with current therapies. New treatment options are needed for patients with relapsed or treatment-resistant (refractory) DLBCL or MCL.
Scientists are studying the drug flavopiridol to see if it can be effective in treating these diseases. Flavopiridol belongs to a class of drugs known as cyclin-dependent kinase (CDK) inhibitors. CDKs are proteins that help control cell proliferation. To be active, CDKs must interact with other proteins called cyclins.
MCL cells are distinguished by an excess of cyclin D1, and scientists believe that blocking the activity of this protein through CDK inhibition
is a potential therapeutic strategy
that may cause MCL cells to die.
In addition, preliminary results
suggest that flavopiridol may be
active against DLBCL.
"Because there are numerous molecular targets for this drug in these diseases, we have a very good scientific rationale for investigating flavopiridol in these lymphomas," said Dr. Dunleavy. "We hope that inhibiting these targets with flavopiridol will cause these tumor cells to undergo apoptosis, or programmed cell death."
Although a different administration schedule of flavopiridol has been tested previously in the treatment of MCL with disappointing results, Dr. Dunleavy noted that this trial is employing a novel method of drug delivery that incorporates both continuous infusion over several hours and a bolus infusion that delivers a large initial pulse of drug.
"Originally developed for and tested in patients with chronic lymphocytic leukemia, where it showed excellent efficacy, this hybrid schedule of administration aims to achieve levels of flavopiridol that can effectively kill lymphoma cells," Dr. Dunleavy said.
For More Information
See the list of eligibility criteria and contact information at http://cancer.gov/clinicaltrials/NCI-07-C-0081 or call the NCI Clinical Trials Referral Office at 1-888-NCI-1937. The call is toll free and confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |
Following are newly released NCI research funding opportunities:
Research Supplements to Promote Diversity in Health-Related Research
Announcement Number: PA-08-190
Application Receipt Dates: Applications may be submitted at any time until September 30, 2011.
This is a renewal of PA-05-015 and provides research supplements to existing awards. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3897. Inquiries: Dr. Peter Ogunbiyi - ogunbiyp@mail.nih.gov
Research Supplements to Promote Re-Entry into Biomedical and Behavioral Research Careers
Announcement Number: PA-08-191
Application Receipt Dates: Applications can be submitted at any time until September 30, 2011.
This is a renewal of PA-04-126 and provides research supplements to existing awards. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3898. Inquiries: Dr. Peter Ogunbiyi - ogunbiyp@mail.nih.gov
Molecular Libraries Screening Instrumentation
Announcement Number: RFA-RM-08-020
Letter of Intent Receipt Date: Sept. 2, 2008
Application Receipt Date: Oct. 2, 2008
This funding opportunity will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3901. Inquiries: Dr. Ajay - ajaydr@mail.nih.gov
Research on Causal Factors and Interventions that Promote and Support the Careers of Women in Biomedical and Behavioral Science and Engineering
Announcement Number: RFA-GM-09-012
Letter of Intent Receipt Date: Sept. 21, 2008
Application Receipt Date: Oct. 22, 2008
This funding opportunity will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3900. Inquiries: Dr. Juliana M. Blome - blomeju@mail.nih.gov
Geographic and Contextual Influences on Energy Balance-Related Health Behaviors
Announcement Number: PA-08-192
Application Receipt Dates: Non-AIDS Applications (new): Oct. 5, 2008; Feb. 5, June 5, and Oct. 5, 2009; Feb. 5, June 5, and Oct. 5, 2010; Feb. 5, and June 5, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, and Sept. 7, 2011
This funding opportunity will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3899. Inquiries: Dr. David Berrigan - berrigad@mail.nih.gov
Geographic and Contextual Influences on Energy Balance-Related Health Behaviors
Announcement Number: PA-08-193
Application Receipt Dates: Non-AIDS Applications (new): Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, and June 16, 2011
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, and Sept. 7, 2011
This funding opportunity will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3899. Inquiries: Dr. David Berrigan - berrigad@mail.nih.gov
Measures and Determinants of Smokeless Tobacco Use, Prevention, and Cessation
Announcement Number: RFA-CA-08-024
Letter of Intent Receipt Date: Oct. 24, 2008
Application Receipt Date: Nov. 24, 2008
This funding opportunity will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3896. Inquiries: Dr. Mark Parascandola - paramark@mail.nih.gov
Measures and Determinants of Smokeless Tobacco Use, Prevention, and Cessation
Announcement Number: RFA-CA-08-025
Letter of Intent Receipt Date: Oct. 24, 2008
Application Receipt Date: Nov. 24, 2008
This funding opportunity will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3896. Inquiries: Dr. Mark Parascandola - paramark@mail.nih.gov
|
 |
 |

Training the Next Generation of Biomedical Scientists
It is axiomatic that basic biomedical research is an engine that drives advances in the prevention and treatment of human disease. Our increasingly detailed knowledge of cell physiology, coupled with new methods of analysis, has placed demand on the scientific community to rapidly translate bench findings to the clinic. Yet the training necessary to master all elements of this most important of endeavors is fragmented, with curricula for the Ph.D. and M.D. degrees being distinctly different.
 The traditional solution to this problem, studying for both degrees, requires considerable personal investment. With our Ph.D. Program in Cancer Biology (CBGP) at the Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan-Kettering Cancer Center (known as Gerstner Sloan-Kettering, or GSK), we are trying to create a new kind of pathway to translational research - one that does not require both M.D. and Ph.D. degrees.
The traditional solution to this problem, studying for both degrees, requires considerable personal investment. With our Ph.D. Program in Cancer Biology (CBGP) at the Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan-Kettering Cancer Center (known as Gerstner Sloan-Kettering, or GSK), we are trying to create a new kind of pathway to translational research - one that does not require both M.D. and Ph.D. degrees.
The CBGP at GSK offers training in the biomedical sciences through the lens of a human disease, cancer. The program is designed to produce Ph.D. graduates who have a solid grounding in basic biomedical science, as well as significantly increased exposure to clinical research. The long-term goal is to train investigators who can serve as intellectual bridges to connect these two biomedical research communities.
To achieve this, we have developed a curriculum that integrates the basic and clinical sciences during the first year of graduate school. The primary didactic experience is the Core Course, which meets daily. During the first year, students also shadow physicians in the clinic to develop an appreciation for the human side of cancer and meet with clinicians of all ranks to begin to develop an appreciation for the challenges faced in developing effective treatments.
The classroom exercises start with genes and proteins and end with clinical issues, and the classes are taught by basic scientists, physician scientists, and clinicians. A typical day includes both a lecture and group discussion of a paper. Many topics lend themselves to the kind of integration that we seek. For example, the treatment of DNA topoisomerases starts with my lecture on the basic biochemistry of the enzymes and the molecular mechanism of topoisomerase inhibitors, such as the anticancer drugs etoposide and topotecan. My colleague Dr. David Spriggs, the head of the Division of Solid Tumor Oncology in the Department of Medicine at Memorial Hospital, then follows with a lecture on the use of anti-topoisomerase drugs in the clinic, providing a gateway to a more general discussion of chemotherapeutic agents. In addition, the students read and discuss a paper from Dr. Leroy Liu's group describing the seminal observation that DNA replication is required for the activity of camptothecin. One important aspect of the group discussion is how this observation should be factored into treatment, weighing both benefit and toxicity.
After choosing a thesis laboratory and completing a thesis proposal, students can continue to learn about clinical research through a clinical mentor who is a member of the attending staff. Selection of clinical mentors is guided by a student's research project. For example, a student studying meiosis might have a clinical mentor who studies and treats patients with germ cell tumors. The clinical mentor serves as a student's conduit to hospital-based academic activities, such as grand rounds, resident's reports, and core lecture series.
The goal of this clinical apprenticeship, which has both formal and tutorial aspects, is to encourage students to develop a clinical perspective on the application of bench work to
the clinic. During this process, we expect our students to gain facility with the clinical lexicon, familiarity with the workings of clinical trials, understanding of the difficulties of proving efficacious treatments, an appreciation of how human physiology can dictate the mode of intervention, and knowledge of the various
mechanisms and technologies that exist for the development of new, innovative treatments.
The CBGP at GSK is in its formative years, so we are still a work in progress. But the remarkably gratifying response from the pool of students applying to graduate school suggests both a need and desire for programs such as ours.
Dr. Kenneth J. Marians
Dean, Louis V. Gerstner Jr.
Graduate School of Biomedical Sciences, Memorial Sloan-Kettering Cancer Center
|
 |
 |
 Summit Focuses on the Science of Cancer Health Disparities
Summit Focuses on the Science of Cancer Health Disparities
NCI's Center to Reduce Cancer Health Disparities (CRCHD) held the third annual Cancer Health Disparities Summit July 14-16 in Bethesda, MD. The theme of Summit 2008 was "Eliminating Cancer Health Disparities Through Science, Training, and Community."
"We know that many populations in the United States suffer disproportionately from cancer," said CRCHD Director Dr. Sanya Springfield.
"I'm pleased to report that much is being accomplished and much progress is being made to bring new advances in cancer research to our African American, Latino, Native American, Asian, Native Hawaiian/Pacific Islander, and medically underserved communities."
The Summit attracted over 900 cancer researchers, health professionals, and community health educators involved nationwide in disparities research, training, education, and outreach programs. During the Summit, four ongoing CRCHD initiatives were highlighted: Community Networks Program (CNP), Continuing Umbrella of Research Experiences (CURE), Minority Institution/Cancer Center Partnership Program (MI/CCP), and the Patient Navigation Research Program (PNRP).
Over the 3 days, participants
shared their ideas and their science, discussed successes, as well as problems, learned from each other, and networked.
"It was an exciting couple of days," said Dr. Springfield, "and it was tremendously gratifying to see firsthand the drive and commitment from this phenomenal body of researchers, clinicians, and community members who make a difference in the lives of our cancer patients and our communities.
"The Summit was a tremendous success," she continued. "However, we know we can always do better, and we continue to look to the community to help us in our efforts to reduce and ultimately eliminate cancer health disparities."
NCI Hosts Science Writers' Seminar on Public/Private Partnerships
On July 28, the NCI Office of Media Relations will host a science writers' seminar to discuss public/private partnerships in cancer research. Topics will include working with industry to develop new drugs; cancer vaccine development and business barriers; working with small businesses to develop new technologies to catch cancer in its earliest stages; and intellectual property rights and technology transfer issues related to new genetic tests.
Leading experts from NCI will participate in the seminar, including NCI Director Dr. John E. Niederhuber, Dr. James Doroshow, Dr. Jeffrey Schlom, Mr. Michael Weingarten, Ms. Karen Maurey, and Dr. Jason Cristofaro.
To view a live Webcast of the seminar at 9:00 a.m. on July 28, or to view an archived video at a later date, please go to http://videocast.nih.gov/summary.asp?live=6921.
Latest Issue of DCEG Newsletter Available Online
The July 2008 issue of Linkage, a newsletter published three times a year by NCI's Division of Cancer Epidemiology and Genetics, is
available online.
Readers will find articles on DCEG's biennial Molecular Epidemiology Course, Dr. Ola Landgren's research on multiple myeloma, Dr. Amanda Cross' work examining meat intake and cancer risk, scientific highlights by DCEG investigators, and the latest news from the division.
To view the issue, go to http://dceg.cancer.gov/newsletter/Linkage.html.
| |
 |
 |

Burnham Institute for Medical Research Cancer Center
Director: Dr. Kristiina Vuori • 10901 North Torrey Pines Road, La Jolla, CA 92037 • Phone: 858-646-3100 • Web site: http://www.burnham.org/default.asp?contentID=28
 Background
Background
Founded in 1976 as the La Jolla Cancer Research Foundation, Burnham Institute for Medical Research (Burnham) is dedicated to finding new ways to fight cancer and other diseases, guided by the belief that the most substantial breakthroughs in fighting disease come from basic scientific inquiries into the inner workings of cells and their related molecules. The Institute is highly collaborative, merging the talents of biologists with chemists, biophysicists, engineers, and computer scientists to tackle today's great unmet medical challenges.
Burnham is home to one of the leading basic cancer research centers in the world, focused on fundamental research into the molecular properties of cancer. Burnham has been an NCI-designated Cancer Center since 1981, one of only seven such basic research centers in the nation. Burnham's Cancer Center includes programs in: tumor microenvironment, tumor development, signal transduction, and apoptosis and cell death research.
Research
The Cancer Center mobilizes more than 400 staff who work to stop cancer before it develops, detect cancer at its earliest point, and eliminate cancer's deadly spread. Other major efforts include developing targeting technologies that deliver anticancer drugs directly to tumors, thereby avoiding serious side effects, and technologies to trick cancer cells into committing suicide by restoring the body's natural mechanisms for cell death.
The institute is bridging the gap between basic biological research, in which Burnham has traditionally excelled, and efforts to identify new targets for cancer therapies. Burnham's Cancer Center has received one of only nine NCI National Cooperative Drug Discovery Group grants. With these resources, Burnham will search for new targets for cancer therapies, looking at the molecules that cancers depend on for growth and survival.
Other Programs
In addition to the NCI-designated Cancer Center, Burnham has four other centers which include the Del E. Webb Neuroscience, Aging and Stem Cell Research Center; the Infectious and Inflammatory Disease Center; the Diabetes and Obesity Research Center (headquartered at Burnham's Orlando, FL facility); and the Sanford Children's Health Research Center.
These disease-focused centers are supported by an underlying research infrastructure, which provides sophisticated technologies and services to enhance scientific discovery. These core facilities are supported largely by special grants from NIH, including an NIH Blueprint grant that establishes Burnham as the lead organization for one of the nation's first two Centers for Neuroscience and Stem Cell cores. Research is further bolstered by several technology-focused centers that provide specialized support in chemical genomics, proteomics, stem cells, computational modeling and vascular mapping, and bionanotechnology.
|
 |
|