Bill & Melinda Gates Foundation awards $168.7 million to PATH for its Malaria Vaccine Initiative
Award will accelerate development of a new generation of malaria vaccines. Read more»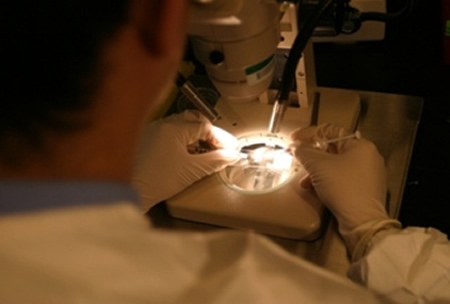
Partnering with us
We are pursuing a range of vaccine development approaches with an expanding circle of industry, academic, and government partners.
Our portfolio
 We support a diversity of malaria vaccine candidates at different stages of development.
We support a diversity of malaria vaccine candidates at different stages of development.
What we do and how we do it
MVI provides funding and technical support to partners worldwide to accelerate the development of malaria vaccines. MVI also works to ensure that malaria vaccines will reach those who need them.
Find out more»
Photo: Sanaria Inc.



