 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Incidence of Pap Test Abnormalities Within 3 Years of a Normal Pap Test --- United States, 1991--1998Declines in cervical cancer incidence and mortality reported in the United States since the 1950s have been attributed to early detection and treatment of precancerous and cancerous lesions through the use of the Papanicolaou (Pap) test (1). More than 50 million Pap tests are performed each year (2); however, guidelines about the frequency of testing in women with a history of normal test results are inconsistent (3--5). To determine the incidence of cervical cytologic abnormalities following a normal Pap test, 1991--1998 data from the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) were analyzed for this report (6). The findings indicated that within 3 years of a normal Pap test result, severe cytologic abnormalities were uncommon, and incidence rates were similar among women screened 1, 2, and 3 years following a normal Pap test. For each woman, CDC received a report that included demographic characteristics, Pap test results, diagnostic procedures, and histopathologic results (6,7). To be eligible for the analysis, women were required to have had a first NBCCEDP Pap test reported as normal during 1991--1998, and at least one subsequent Pap test performed within the following 9--36 months. Of 620,063 women tested during 1991--1998, 128,805 (20.8%) met the criteria for eligibility. Results of Pap tests were reported using Bethesda System categories: normal; infection, inflammation, or reactive changes; atypical squamous cells of undetermined significance (ASCUS); low-grade squamous intraepithelial lesion (LSIL); high-grade squamous intraepithelial lesion (HSIL); "suggestive of squamous cell carcinoma"; and "other" (e.g., glandular atypia and atypical endocervical glands). Incidence rates of Pap test interpretations were calculated by dividing the number of women with each test result by the number of women retested within each age group (<30, 30--49, 50--64, and >65 years) and time interval (9--12, 13--24, and 25--36 months). Incidence rates were age-adjusted using the age distribution of the 1996 NBCCEDP population. Ordinary least-squared regression was used to evaluate the trend of increasing time between the first Pap test on the age-adjusted incidence of ASCUS, LSIL, HSIL, and suggestive of squamous cell carcinoma. The average age of women included in the analysis was 48.9 years (range: 12--96 years); 73,631 (57.0%) were non-Hispanic whites, 22,672 (17.6%) were Hispanics, 17,314 (13.4%) were non-Hispanic blacks, 10,983 (8.5%) were American Indians/Alaska natives, 3070 (2.4%) were Asians/Pacific Islanders, and 1135 (0.9%) were categorized as "other" or "unknown." The mean time between the first and second test was 15.7 months. Approximately 121,576 (94.4%) of the 128,805 second test results were interpreted as normal or infection, inflammation, or reactive changes. The incidence rate of the second test results interpreted as HSIL and suggestive of squamous cell carcinomas was 66 per 10,000 women aged <30 years, 22 per 10,000 women aged 30--49 years, 15 per 10,000 women aged 50--64, and 10 per 10,000 women aged >65 years (trend test, p<0.001). Overall, as age increased, the incidence of ASCUS and LSIL also decreased (trend test, p<0.001, each category). The age-adjusted incidence of results interpreted as LSIL increased over time (trend test, p=0.01) (Table 1). The incidence of ASCUS, the most common cytologic abnormality, did not change significantly over time (p=0.36). The differences in the age-adjusted incidence of HSIL and suggestive of squamous cell carcinoma for the time intervals also were not significant (p=0.42). Reported by: GF Sawaya, MD, K Kerlikowske, MD, G Gildengorin, PhD, AE Washington, MD, Univ of California, San Francisco. Div of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion; CDC. Editorial Note:The U.S. Preventive Services Task Force recommends Pap test screening at least every 3 years until age 65 years (5). The American Cancer Society guidelines suggest that screening less frequent than annually may be adequate for Pap testing in women with a history of 3 negative annual Pap tests (3), and the American College of Obstetricians and Gynecologists recommends annual Pap tests for most women (4). The difference in screening annually, biennually, or triennially is substantial in the number of tests performed and in the public health implications. In this analysis, women screened 1, 2, and 3 years after a normal Pap test had similar risk for developing HSIL and suggestive of squamous cell carcinoma. Other studies have indicated clinically insignificant additional protection in testing yearly compared with triennially (8). However, low-grade abnormal Pap results (e.g., ASCUS and LSIL) constituted >95% of the cytologic abnormalities after the first normal results. The clinical significance of these abnormalities is unclear. Women who were screened annually rather than less frequently might have worse health outcomes if low-grade results of undetermined clinical importance lead to further testing and unnecessary patient morbidity and anxiety (9,10). The findings in this report are subject to at least four limitations. First, the database used was intended for descriptive statistics and not for hypothesis testing; data were limited to a few variables. Second, NBCCEDP serves low-income and uninsured women; results may not be generalizable to other groups. However, low-income and uninsured women usually are at greater risk for developing cervical neoplasia than women with higher incomes; therefore, higher-income women should be less likely to exhibit higher rates during the 3-year interval examined in this study. Third, women may have received Pap testing outside the program during the time between the first and subsequent Pap tests; however, this probably occurred in only a few women. Finally, women who frequently get screened, specifically within 1 year after Pap test, might be low-risk women concerned about their health or high-risk women with histories of abnormal Pap tests who have been told to get annual tests. Other risks for cervical cancer in these women and whether these risks affected the findings in this study are unknown. NBCCEDP receives data from many cytopathology laboratories and clinical settings. The findings in this study may better represent actual clinical settings than the findings in a controlled trial. CDC is working with state health departments to use this information as a basis for cost-effective strategies to reach women who have not received screening services for cervical disease. CDC will assist NBCCEDP in assessing program-provider practices, modifying patient recall systems, and developing professional and public education strategies to improve patient-provider decision making. Further research is needed to clarify the benefit and harm related to frequency of subsequent Pap testing in women with normal results. References
Table 1 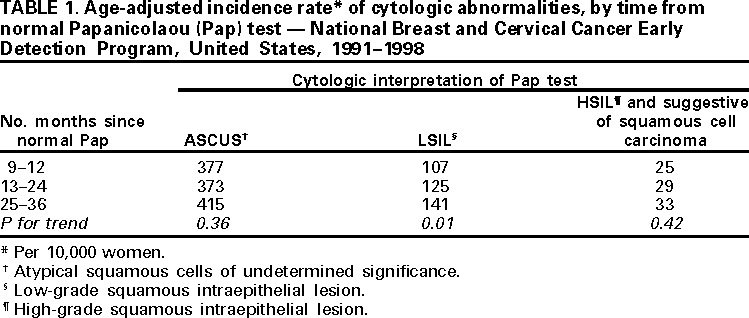 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/9/2000 |
|||||||||
This page last reviewed 5/2/01
|