 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Progress Toward Poliomyelitis Eradication --- India, 2003Since the World Health Assembly resolved in May 1988 to eradicate poliomyelitis, the estimated global incidence of polio has decreased >99%, and three World Health Organization (WHO) regions (Americas, Western Pacific, and European) have been certified as polio-free (1). Since 1994, when the countries of the WHO South-East Asia Region (SEAR)* began accelerating polio-eradication activities, substantial progress toward that goal has been made (2--4). By 2001, poliovirus circulation in India had been limited primarily to the two northern states of Uttar Pradesh and Bihar, with 268 cases reported nationwide. However, a major resurgence of polio occurred during 2002, with 1,600 cases detected nationwide, of which 1,363 (85%) were in Uttar Pradesh and Bihar (5). This report summarizes the status of polio eradication activities in India during 2003 and describes the actions being taken to reduce poliovirus transmission. Acute Flaccid Paralysis SurveillanceIn 2003, a network of 248 trained surveillance medical officers (SMOs) assisted local health authorities at the district or subdistrict level with acute flaccid paralysis (AFP) surveillance. Since 2000, India has exceeded the WHO-established AFP surveillance quality targets (i.e., nonpolio AFP rate of >1 per 100,000 population aged <15 years and adequate stool specimens† collected from >80% of persons with AFP) (Table). However, during 2003, the nonpolio AFP rate was <1 per 100,000 in seven small states (Chandigarh, Dadra and Nagar Haveli, Lakshadweep, Manipur, Mizoram, Nagaland, and Tripura) with approximately 1% of India's total population, and inadequate (70%--80%) stool specimen collection was reported in 11 states (Bihar, Chhattisgarh, Dadra and Nagar Haveli, Daman and Diu, Delhi, Lakshadweep, Madhya Pradesh, Mizoram, Sikkim, Uttaranchal, and Uttar Pradesh) with approximately 35% of India's total population. Wild Poliovirus IncidenceDuring 2003, a total of 225 wild poliovirus (WPV) cases were reported§ from India, a substantial decrease from the 1,600 cases reported in 2002 (Table). Of these 225 cases, 203 (90%) were WPV type 1 (P1), and 22 (10%) were WPV type 3 (P3). During 2003, incidence decreased substantially in the three states that had the highest number of cases in 2002: from 1,242 to 88 in Uttar Pradesh, from 121 to 18 in Bihar, and from 49 to 28 in West Bengal. However, new foci of disease were reported in the southern Indian states of Karnataka (36), Andhra Pradesh (21), and Tamil Nadu (two), each of which had reported no polio cases for >2 years. Cases were reported from 88 (15%) of 587 districts nationwide, compared with 159 districts (27%) in 2002 (Figure 1). P3 circulation occurred primarily in Uttar Pradesh (16 [73%] cases). Of the 88 cases in Uttar Pradesh that were confirmed virologically, 60 (68%) occurred in minority populations, which constitute approximately 17% of the state's total population. During 2002--2003, the number of circulating genetic lineages of WPV remained constant for P1 (n = three) and P3 (n = four). All lineages circulating in India in 2003 were derived from strains that circulated in Uttar Pradesh during 2000--2001. Vaccination CoverageDuring 2002, approximately 68% of infants aged <1 year received >3 doses of oral poliovirus vaccine (OPV) through routine vaccination. Substantial variations by state were found in routine coverage with 3 doses of OPV (OPV3), ranging from 21% in Bihar to 99% in Madhya Pradesh; OPV3 coverage through routine vaccination in Uttar Pradesh was estimated to be 41% (6). Since 1995, biannual national immunization days (NIDs)¶ that use fixed-site vaccination posts to administer OPV have been conducted to supplement routine vaccination and interrupt transmission of WPV. During 1999, supplementary immunization activities (SIAs) were intensified with the addition of house-to-house vaccination after an initial day of fixed-site activities. During 1999--2002, the number of large-scale NIDs and subnational immunization days (SNIDs)** conducted in India decreased, from six during October 1999--March 2000 to four during 2000--2001 and three during 2001--2002 (Figure 2). During 2002--2003, two NIDs and four large SNIDs (the latter targeting 60--70 million children during each round) were conducted. In addition, monitoring of SIA quality was enhanced by the introduction of new vaccinator data-collection forms and standardized independent observer checklists. Data were analyzed to identify areas of programmatic weakness and to focus attention on specific districts and blocks with deficiencies in SIA quality. In Uttar Pradesh and Bihar, vaccination coverage data for AFP cases that were not caused by polioviruses indicate that OPV coverage improved substantially during 2002--2003. The proportion of children aged 6--59 months with nonpolio AFP who had <3 OPV doses (routine or supplemental) decreased from 20% to 6% in western Uttar Pradesh and from 17% to 7% in Bihar. However, during the same period, the proportion of such children increased to >23% in eastern Karnataka and to 10% in Andhra Pradesh. Reported by: Ministry of Health and Family Welfare, Government of India; National Polio Surveillance Project, World Health Organization, India; Dept of Immunization and Vaccine Development, World Health Organization, Regional Office for South-East Asia, New Delhi, India. Dept of Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland. Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Global Immunization Div, National Immunization Program, CDC. Editorial Note:India, the only remaining country in SEAR with ongoing indigenous WPV transmission, made major progress toward elimination of WPV in 2003. The 225 cases reported in 2003 represent the lowest annual number of polio cases in India's history, and the two states (Uttar Pradesh and Bihar) that have accounted for the majority of polio cases in India reported the lowest number of cases ever. The increased number and quality of SIAs and expanded social mobilization activities improved the immunity status of the population in every state in which these actions were taken, leading to a decline in disease rates. Although several cases were reported early in 2003 in Delhi, Gujarat, Haryana, and Rajasthan, no cases were reported from these states during July--December, suggesting cessation of WPV transmission in these areas. The outbreak of disease in the south in 2003 was attributable to an increasing proportion of children with <3 doses of OPV, which allowed spread of WPV once introduced. With intensified SIAs, these states should become polio-free again. All cases of paralytic polio reported in India during 2003 were caused by lineages traceable to WPVs circulating in western Uttar Pradesh, which remains the source of polio that has been introduced to areas of the country that had been polio-free for several months or years. Although cases were reported during 2003 from 16 (46%) of India's 35 states, Uttar Pradesh alone had sustained transmission throughout the year. The elimination of these reservoirs of poliovirus is critical to the success of polio eradication in India. In areas where SIA numbers and quality were enhanced in 2003, OPV coverage increased. OPV coverage also increased among minority populations, reflecting efforts made to address operational and social mobilization gaps. However, in several states in the south where additional SIAs were not conducted, vulnerability to infection with WPV increased. During July--December 2003, large mop-up vaccination campaigns were conducted; the impact of these SIAs will be evaluated by using data on nonpolio AFP cases collected during the next 3 months. During January--May 2004, three NIDs and one SNID are planned. These SIAs will be followed by intensive mop-up activities for any cases identified after April, with two additional NIDs planned for the fall. Each NID will target approximately 165 million children, and each SNID will target approximately 100 million children. Statewide AFP surveillance reviews initiated systematically in 2003 will continue, and the results will be used to fill any remaining gaps in surveillance, ensuring detection of any WPV transmission so that mop-up vaccination can be initiated rapidly. The government of India, WHO, United Nations Children's Fund (UNICEF), Rotary International, CDC, and other partners are providing increased support for this effort through additional personnel and funding. Because of its population size, its geographic location, and the ongoing threat of importation of WPV into polio-free countries, eliminating polio from India is the greatest challenge facing the global polio-eradication effort. With fewer cases reported in 2003 than ever before during the traditional high season (June--December), India is close to eliminating WPV transmission nationally. For this effort to succeed in 2004, sustained and effective commitment of national and state governments is required, along with continued support by India's major international partners. References
* Bangladesh, Bhutan, Democratic People's Republic of Korea, India, Indonesia, Maldives, Mongolia, Myanmar, Nepal, Sri Lanka, and Thailand. † Two specimens collected >24 hours apart, both within 14 days of paralysis onset and shipped properly to the laboratory. § Data as of February 28, 2004. ¶ Nationwide mass campaigns during a short period (days to weeks) in which 2 doses of OPV are administered to all children (usually aged <5 years), regardless of previous vaccination history, with an interval of 4--6 weeks between doses. ** Mass campaigns same as NIDs but limited to parts of a country.
Figure 1 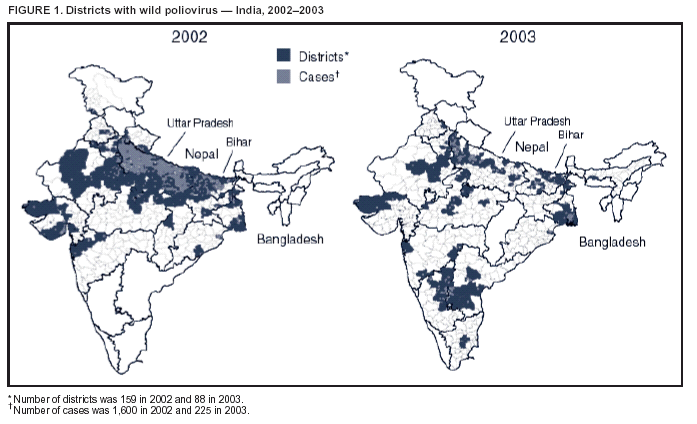 Return to top. Figure 2 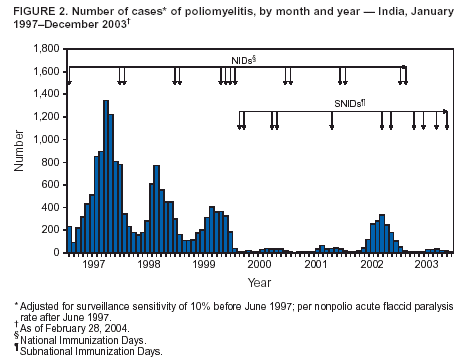 Return to top. Table 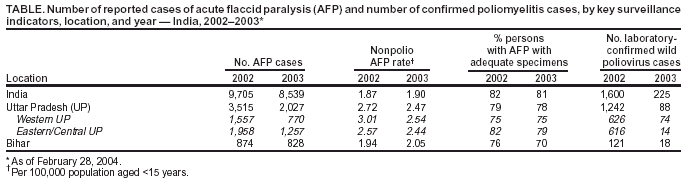 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/25/2004 |
|||||||||
This page last reviewed 3/25/2004
|