 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Update: Adverse Events Following Civilian Smallpox Vaccination --- United States, 2003Please note: An erratum has been published for this article. To view the erratum, please click here. During January 24--December 31, 2003, smallpox vaccine was administered to 39,213 civilian health-care and public health workers in 55 jurisdictions to prepare the United States for a possible terrorist attack using smallpox virus. This report updates information on vaccine-associated adverse events among civilians vaccinated since the beginning of the program and among contacts of vaccinees, received by CDC from the Vaccine Adverse Event Reporting System (VAERS) during August 9--December 31. In this vaccination program, CDC, the Food and Drug Administration, and state health departments are conducting surveillance for vaccine-associated adverse events among civilian vaccinees (1,2). As part of the vaccination program, civilian vaccinees receive routine follow-up, and reported adverse events after vaccination receive follow-up as needed. The U.S. Department of Defense is conducting surveillance for vaccine-associated adverse events among military vaccinees and providing follow-up care to those persons with reported adverse events (3). Adverse events associated with smallpox vaccination are classified on the basis of evidence supporting the reported diagnoses. Cases verified by virologic testing or, in some instances, by other diagnostic testing, are classified as confirmed (Table 1). Cases are classified as probable if possible alternative etiologies are investigated and excluded and supportive information for the diagnosis is found. Cases are classified as suspected if they have clinical features compatible with the diagnosis, but either further investigation is required or investigation of the case did not provide supporting evidence for the diagnosis. All reports of events that follow vaccination (i.e., events associated temporally) are accepted; however, reported adverse events are not necessarily associated causally with vaccination, and some or all of these events might be coincidental. This report includes cases reported as of December 31 that are either under investigation or have a reported final diagnosis. During August 9--December 31, no new cases of selected adverse events were reported (Table 1). During the vaccination program, no cases of eczema vaccinatum, erythema multiforme major, fetal vaccinia, or progressive vaccinia have been reported. During August 9--December 31, a total of 20 other serious adverse events were reported (Table 2). Also during this period, 59 other nonserious events were reported. Among the 712 vaccinees with reported other nonserious adverse events during January 24--December 31 (Table 2), the most common signs and symptoms were rash (n = 142), fever (n = 135), pain (n = 122), headache (n = 111), and fatigue (n = 97). All of these commonly reported events are consistent with mild expected reactions following receipt of smallpox vaccine. Some vaccinees reported multiple signs and symptoms. During this reporting period, no vaccinia immune globulin was released for civilian vaccinees. No cases of vaccine transmission from civilian vaccinees to their contacts have been reported during the vaccination program (Table 3). Surveillance for adverse events during the civilian and military smallpox vaccination programs is ongoing. Reported by: Smallpox vaccine adverse events coordinators; National Immunization Program, CDC. References
Table 1 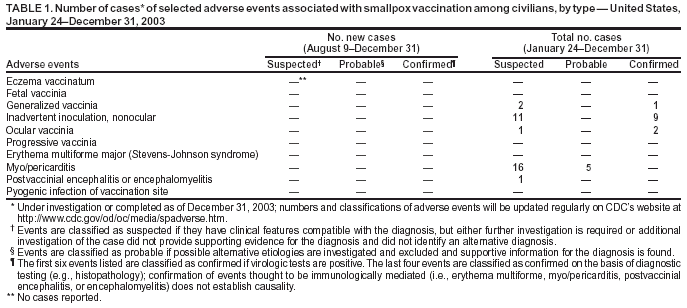 Return to top. Table 2 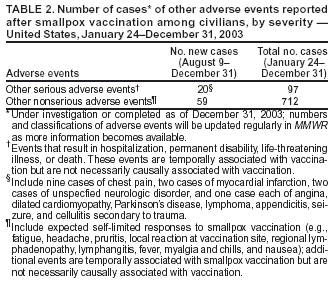 Return to top. Table 3 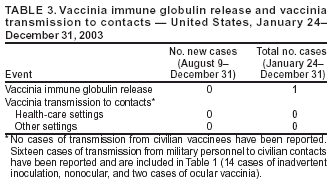 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 2/12/2004 |
|||||||||
This page last reviewed 2/12/2004
|