 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Measles Outbreak Associated with an Imported Case in an Infant --- Alabama, 2002Local transmission of measles is rare in the United States. Since 1997, the majority of measles outbreaks have been caused by imported* cases (1). During October 19--November 15, 2002, an outbreak of 13 confirmed† cases of measles occurred, with exposure in Alabama; 11 cases were among day care attendees who had not yet been vaccinated for measles. This was the largest outbreak of measles in the United States since 1999 (2). In response to this outbreak, the Alabama Department of Public Health (ADPH) and CDC conducted an epidemiologic investigation that determined the outbreak was initiated by an imported case in an infant aged 9 months who had returned recently from the Philippines. Health-care providers should continue to include measles in differential diagnoses for febrile rash illnesses in infants, particularly those with recent travel to areas where measles is endemic. On November 3, 2002, a consulting physician suspected measles in three infants aged 10 months who had been hospitalized with rash onsets during October 28--31; all three infants had attended the same day care center and shared the same room. ADPH confirmed measles in these infants and identified two additional cases in the infant aged 9 months and in another infant aged 10 months, both of whom also shared the same day care center room. The infant aged 9 months was hospitalized during October 19--23 and had an initial diagnosis of dengue fever, later reclassified as a fever of unknown origin. The infant had been administered immunoglobulin before departure from the United States to the Philippines. Measles eventually was confirmed in all five infants by enzyme-linked immunosorbent assay testing. Outbreak investigators identified and interviewed persons who had been in contact with infants confirmed with measles during the 4 days before and after rash onset. An additional eight cases were identified among six infants and two adults (Figure). All 11 infants attended the same day care center and shared the same room (attack rate: 100%). Among the 11 infants, the median age was 11 months (range: 10--13 months); seven (64%) were female. The two adults, a man aged 31 years and a woman aged 50 years, were exposed to the same infant. The man had visited the infant's home before hospitalization, and the woman provided nursing care to the infant during hospitalization. All 13 patients had rash, fever, coryza, and cough; 12 (92%) had conjunctivitis, and four (31%) were hospitalized. Nasopharyngeal and/or urine samples were collected from 10 patients (both adults and eight infants); all were positive for measles by viral culture or by polymerase chain reaction. Viral isolates were identified as the D3 measles genotype, known to be circulating in the Philippines (3). Among the 11 infants, none had been vaccinated with a measles-containing vaccine (MCV). None of the infants' mothers reported ever having measles; however, all mothers had been vaccinated with >1 dose of MCV. Among the adults, the man had been vaccinated with 2 doses of MCV before his exposure; the woman had never been vaccinated for measles, although she knew she had a negative titer. ADPH conducted contact tracing and identified 679 persons with known contact with the patients; 616 (91%) were exposed before ADPH was notified. ADPH determined whether exposed persons were ill, assessed vaccination status and recommended measles vaccine, and instructed contacts to monitor for fever during the 18 days after exposure to a patient. If fever occurred during this period, ADPH instructed contacts to isolate themselves and notify their doctors and local health departments. All contacts were considered susceptible unless they had documentation of adequate vaccination, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. Households were called every other day to ask about fever status. ADPH alerted all physicians in the affected county and provided free measles, mumps, and rubella (MMR) vaccine to attendees of the affected day care center and to the public. ADPH recommended that the day care center exclude infants with febrile rash illness until measles was ruled out in a suspected infant. ADPH also recommended a first dose of MMR for day care attendees aged 6--11 months, followed by the regular MMR 2-dose series starting at age 12--15 months (4). In addition, ADPH recommended a first dose of MMR for nonvaccinated infants aged 12--15 months and a second dose of MMR for infants aged >12 months who had a first dose at least 4 weeks previously. Reported by: JP Lofgren, MD, V Cochran, O Abbott, C Woernle, MD, Alabama Dept of Public Health. JM Hayes, DrPH, Div of Applied Public Health Training, Epidemiology Program Office, CDC. Editorial Note:The findings in this report illustrate the high transmissibility of measles when the virus is introduced into susceptible populations. The infant with imported measles and nine infant contacts who had measles were not in an age group recommended to receive an MCV. The Advisory Committee on Immunization Practices (ACIP) recommends that children receive their first MMR dose at age 12--15 months (4). The findings also highlight the need for health-care workers to follow ACIP guidelines to receive 2 doses of MCV or have proof of positive measles titer (5). High immunity levels and effective control measures helped limit the spread of measles in this outbreak. Among Alabama children born during February 1998--May 2000, approximately 94% had >1 dose of MCV (6). ADPH efforts to limit exposure (<10% of contacts occurred after instituting control measures), to educate clinicians and the public about this outbreak, and to increase vaccination services in the affected county also might have helped limit measles transmission. To ensure prompt measles diagnoses, physicians who care for children need to be familiar with the clinical signs of measles. To protect infants against measles, physicians should consider administering MMR vaccine to children aged >6 months who will be traveling outside of the United States and administer it >14 days before administering immunoglobulin. Measles should be included in differential diagnoses for febrile rash illnesses in infants, particularly among those with recent travel to endemic areas. Physicians should report measles cases promptly to their state or local health departments. Acknowledgments Georgia Div of Public Health. Div of Applied Public Health Training, Epidemiology Program Office; Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Div of Epidemiology and Surveillance, National Immunization Program, CDC. References
* Acquired outside the United States, Guam, Puerto Rico, and the U.S. Virgin Islands.
Figure 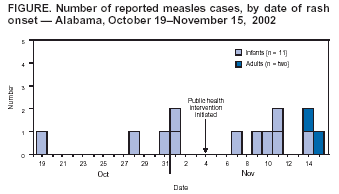 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/22/2004 |
|||||||||
This page last reviewed 1/22/2004
|