 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Progress Toward Elimination of Perinatal HIV Infection --- Michigan, 1993--2000In 1994, the U.S. Public Health Service (PHS) issued guidelines for maternal and neonatal zidovudine (ZDV) use to reduce perinatal human immunodeficiency virus (HIV) transmission (1). These guidelines recommend maternal ZDV use during the second and third trimesters of pregnancy and during labor and delivery (L&D) and administration of ZDV to the neonate for the first 6 weeks of life. In 2001, PHS updated 1995 guidelines for routine HIV counseling and voluntary testing of pregnant women (2,3). The Michigan Department of Community Health (MDCH) requires reporting of all children who are perinatally exposed to HIV and follows up these children to monitor their infection status and record demographic, clinical, and laboratory characteristics of infected children. The reporting of perinatally HIV-exposed children enables MDCH to monitor the effectiveness of public health efforts to prevent perinatal HIV transmission (4) and assists the targeting of prevention programs and activities. This report summarizes surveillance data collected through December 31, 2001, on children born to HIV-infected women in Michigan during 1993--2000. The report highlights rapid adoption of PHS guidelines that resulted in the reduction of perinatally acquired HIV infection to historically low levels in Michigan. Improving levels of prenatal care (PNC) for HIV-infected pregnant women, especially substance users, and routine HIV counseling and voluntary testing for all pregnant women are needed to further reduce perinatal HIV infection. MDCH collects testing and treatment data on all children born to HIV-infected mothers through routine completion of case reports by state health department staff in cooperation with health-care providers, hospitals, and clinics. To ensure complete reporting of mother-infant pairs and to identify possible factors that can improve outcomes for HIV-infected mothers and their infants, additional case ascertainment and public health follow-up activities are conducted. To identify recent births to HIV-infected women who were previously reported as having HIV infection or acquired immunodeficiency syndrome (AIDS), the Michigan HIV/AIDS Registry (HARS) was matched to the Michigan Birth Registry for birth years 1993 through 1999 using standard matching algorithms. Maternal records (i.e., PNC, clinic, and L&D records) and pediatric records (i.e., birth and clinic records) were reviewed to complete and supplement information collected on the routine case-report form. Timing of maternal HIV testing, number of PNC visits received, maternal use of alcohol and illegal drugs during the most recent pregnancy, and the frequency of sexually transmitted disease (STD) diagnoses during pregnancy were abstracted from available medical records. For birth years 1993--2000, data were abstracted for 512 mother-infant pairs and for six HIV-exposed infants for whom maternal information was unavailable. The HARS-birth registry match identified 39 (8%) of these HIV-exposed children. For birth years 1993 and 1994 combined, the case ascertainment methods identified 146 (95%) of 153 perinatally exposed infants when compared with available data for that period from the Survey of Childbearing Women, an anonymous serologic survey of the presence of maternal antibodies in all newborns. Maternal ZDV use prenatally and/or during L&D increased significantly from 27% in 1993 to 85% in 2000 (p<0.01; chi square for linear trends) (Table 1), and peaked at 95% in 1998. Neonatal ZDV use increased from 12% in 1993 to 93% in 2000 (p<0.01; chi square for linear trends). Of six women who refused ZDV treatment during both pregnancy and L&D, five gave birth before 1996, and four of their infants received neonatal ZDV. The percentage of mothers who received other antiretroviral medications in addition to ZDV during pregnancy increased from 5% in 1993 to 71% in 2000. On the basis of follow up of children for at least 12 months, the number of children known to be perinatally HIV-infected decreased from 19% to 3% from 1993 to 2000 (p<0.01; chi square for linear trends). Although there has been insufficient follow-up time to determine infection status definitively for children born in 1999 and 2000 who are of indeterminate status, most had one negative polymerase chain reaction test before age 4 months (5) and are not likely to be infected. Medical records were reviewed for 488 HIV-infected women who gave birth during 1993--2000. Of these women, information on receipt of PNC was missing for 57 (12%) (Table 2). Of the 431 women with documented PNC information, 45 (10%) received no PNC. Overall, 49% of women were tested for HIV before their most recent pregnancy. Of women who had zero PNC visits, 58% had been tested before or during their most recent pregnancy compared with 94% and 93% who had 1--2 and >3 PNC visits, respectively. Additional information on illegal drug use, alcohol, and STDs was available on 344 (80%) of these women (Table 2). Of these, drugs and alcohol were used more frequently by women who had zero or 1--2 PNC visits, compared with those who had >3 (chi square p=0.02 and p=0.06, respectively). For all categories of PNC care, cocaine and/or crack were the most frequently used illegal drugs (62%). A higher proportion of women who had >3 PNC visits were diagnosed with one or more STD (gonorrhea, chlamydia, syphilis, primary genital herpes, pelvic inflammatory disease, and trichomonas) (49%), compared with women with <3 visits (26%) (p=0.09; chi square). To allow time for health-care providers to adopt the 1994 PHS guidelines for ZDV use and to examine their impact on perinatal HIV transmission, the characteristics and infection status of children born during 1995--2000 were examined. Of the 381 perinatally HIV-exposed children born during these years, 31 (8%) became HIV-infected (Table 1). Of these, nine (29%) were reported with AIDS by January 1, 2002. Information on maternal prenatal testing and care was available for 27 (87%) of the infected children. A PNC visit included any clinic (e.g., obstetric or medical) visit at which PNC was provided and excluded visits to emergency departments. Of the 27 mothers for whom PNC information was obtained, four (15%) mothers of HIV-infected children had no PNC visits, four (15%) had 1--2 visits, and 17 (63%) had >3 (mean: 8.4 visits); two had an unknown number of visits, neither of whom received ZDV either before or during L&D. Of the 17 mothers of infected children who had >3 PNC visits, seven (41%) were not tested for HIV until after L&D. Reported by: ED Mokotoff, MPH, BH Malamud, MPH, JB Kent, MS, RJ Kowalczyk, MPH, LJ Scott, Michigan Dept of Community Health. ML Lindergren, MD, TA Hammett, MPH, Div of HIV/AIDS Prevention--Surveillance and Epidemiology, National Center for HIV, STD, and TB Prevention, CDC. Editorial Note:The findings in this report indicate that a high proportion of health-care providers in Michigan are following PHS guidelines for maternal and neonatal ZDV use to reduce perinatal HIV transmission. Since 1994 in Michigan and in other states, an increasing proportion of pregnant women received HIV counseling and testing and ZDV therapy, resulting in a dramatic decrease in the number of children with perinatally acquired HIV/AIDS (6,7). The use of other antiretrovirals increased following the 1996 introduction of combination highly active antiretroviral therapy (HAART) (8), which lowers maternal HIV viral load and contributes to the decreasing transmission rate. Promoting access to PNC, acceptance of HIV testing, and ZDV use are necessary to sustain these trends and to achieve further reductions. Since 1989, Michigan law has required testing of pregnant women at the time of intitial examination for HIV, hepatitis B, and other STDs unless they do not consent to the test or it is contraindicated. In 1994, Michigan law was expanded to include this voluntary testing at the time of delivery or immediately postpartum if no previous testing is documented in her medical records. To comply with these laws, obstetric providers should offer all pregnant women HIV counseling and voluntary testing regardless of their race, age, or marital or socioeconomic status. The findings in this report indicate that most Michigan obstetric providers who care for HIV-infected women are complying with the law. Despite high rates of compliance, opportunities are being missed for perinatal HIV prevention. When women present for delivery to high-prevalence hospitals without documented HIV test results, counseling and voluntary rapid testing should be provided at L&D and results returned to the patient and her obstetric provider as soon as possible so that, if appropriate, timely initiation of intrapartum antiretrovirals or neo-natal ZDV is possible within 48 hours after birth (9). At lower prevalence hospitals, expedited use of standard EIA tests and rapid turnaround of test results at the time of delivery to allow time for administration of intrapartum and neonatal ZDV for women whose HIV status is unknown might be another way to enhance these efforts. Continued efforts are needed to assist pregnant women to obtain PNC and to provide them with HIV counseling and testing. In Michigan, 10% of HIV-infected women received no PNC, compared with 1% in the general population (10). The high prevalence of STDs and illegal drug and alcohol use among HIV-infected women giving birth in Michigan suggests that medical practitioners need to provide treatment or appropriate care referrals for HIV-infected women to manage their HIV infection, substance abuse, and other co-morbid conditions and to prevent perinatal HIV transmission (1,8). HIV-infected infants continue to be born to women who receive both HAART and the recommended prevention protocol. Factors that might contribute to continued transmission include incomplete adherence to medication regimens, advanced maternal disease stage or high viral load, obstetric factors surrounding L&D, or treatment-resistant virus. The findings in this report are subject to at least two limitations. First, although completeness of reporting was high for HIV-exposed infants in 1993 and 1994 compared with the number of HIV-positive mothers known to have given birth, comparable data on the total number of infected women giving birth are not available for 1995--2000. Second, the HARS-birth registry match cannot account for unreported maternal cases and would fail to properly identify a match for a woman reported to HARS and birth registries with more than two reported surnames. CDC provides funds to 21 states to collect expanded perinatal surveillance data as part of a comprehensive public health effort to further reduce rates of perinatal transmission. In Michigan, pediatric HIV surveillance includes children exposed to HIV perinatally, those with HIV, and those who meet the AIDS case definition (4). The findings in this report underscore the importance of collecting comprehensive perinatal surveillance data for monitoring and evaluating both successes and failures in preventing perinatal transmission of HIV (6). References
Table 1  Return to top. Table 2 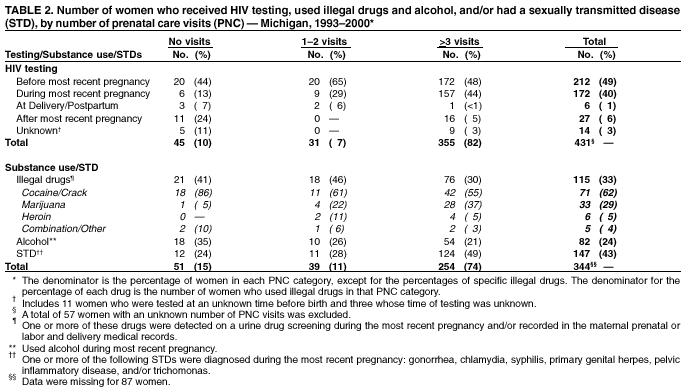 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 2/7/2002 |
|||||||||
This page last reviewed 2/7/2002
|