 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Immunization Registry Use and Progress --- United States, 2001Immunization registries are confidential, population-based, computerized information systems that collect vaccination data about all children within a geographic area (1). Registries are key tools used to increase and sustain high vaccination coverage by providing complete and accurate information on which to base vaccination decisions. Registries consolidate vaccination records of children from multiple health-care providers, identify children who are due or late for vaccinations, generate reminder and recall notices to ensure that children are appropriately vaccinated, and identify provider sites and geographic areas with low vaccination coverage. One of the national health objectives for 2010 is to increase to 95% the proportion of children aged <6 years who participate in fully operational population-based immunization registries (objective 14-26) (2). CDC analyzed data from 50 states and the District of Columbia (DC) from the calendar year 2000 Immunization Registry Annual Report (CY 2000 IRAR) to assess current registry activity. This report summarizes the results of those analyses, which indicate that 32 (63%) of the 51 grantees are operating population-based immunization registries (Figure 1). These 32 projects represent 49% of the U.S. population aged <6 years (3). The CY 2000 IRAR was a self-administered questionnaire distributed to immunization program managers as part of the yearly reporting requirement for Public Health Service Act § 317b grantees. Responses were received from all 50 states and DC. A total of 32 (63%) of the 51 grantees reported operating population-based registries that targeted their entire catchment areas. Of the remaining 19 (37%) grantees, four (21%) reported operating population-based registries in regions or counties as demonstration or pilot projects, and 15 (79%) were planning or developing population-based registries. The CY 2000 IRAR requested information about the percentage of children and vaccination providers in the registry's catchment area who participated and progress in implementing the 13 functional standards for immunization registry operation (3). Data from the 32 grantees operating population-based registries indicated that approximately 49% of the estimated 11.4 million children aged <6 years, based on the U.S. Census, in these catchment areas had >2 vaccinations recorded in the registry. The 32 grantees also reported that an average of 56% of public vaccination provider sites and 41% of private provider sites in their catchment areas participated in the registry during the 6 months preceding completion of the CY 2000 IRAR. All 51 grantees were working to meet key elements of the 13 functional standards established for immunization registries (Table 1). Although no registry has met all key elements of all standards, 14 of the 51 grantees have met all key elements of >11 of the standards (3). Reported by: T Boyd, MS, and RW Linkins, PhD, Data Management Div, National Immunization Program, CDC. Editorial Note:In 2000, the Institute of Medicine noted, "with the increasing importance of population-based approaches to health system planning and evaluation, immunization registries offer one of the most useful instruments for assessing population-specific effectiveness of health and medical care programs" (4). Projects use their registries for program decision support as exemplified by San Antonio's use of registry data to examine the implementation of new vaccines through the Vaccines for Children (VFC) entitlement program. San Antonio's data demonstrated that, although non-VFC providers began using heptavalent pneumococcal conjugate vaccine (PCV7) soon after licensure, delay in VFC reimbursement might have caused slower PCV7 uptake among VFC-eligible children (5). Other projects have used their registry data to measure reminder/recall system effectiveness (6), identify sources of delayed immunizations (7), and track the implementation of immunization schedule changes (8). Oregon registry data were used to assess the impact on hepatitis B (HepB) vaccine administration after issuance of the Joint Statement of the American Academy of Pediatrics (AAP) and the U.S. Public Health Service (USPHS) (9) about using thimerosal as a vaccine preservative. Their joint statement recommended reducing infant exposure to thimerosal; specific recommendations were made to postpone the first HepB vaccine dose until age 2--6 months for infants born to hepatitis B surface antigen-negative mothers. Oregon's registry data (which include 88% of the state's population of children aged <6 years) indicated that the average proportion of children participating in the registry per week who were administered HepB vaccine <5 days after birth decreased 93% during the 6 weeks after the report's release (Figure 2). On the basis of these data, Oregon officials contacted health plans, health-care providers, and local health departments to ensure that the report's recommendations were being followed (i.e., that the first dose of HepB be delayed only for infants born to hepatitis B surface antigen-negative mothers and that providers return to previous infant HepB vaccination practices after a thimerosal-free alternative became available). Continued monitoring of registry data indicated that, despite the availability of thimerosal-free vaccine in August 1999, by the end of 2000, administration rates had reached only 88% of pre-report levels for HepB vaccination (8). These data assist education efforts for providers who have not reinstated HepB vaccine recommendations. CDC recently identified for grantees eight core program attributes in the Immunizations Program Operations Manual released in 2001: population assessment, surveillance, consumer information, service delivery, provider quality assurance, vaccine management, registry development, and program management (10). CY 2000 IRAR data indicate that certain grantees might have the capacity to use registry data to support these program attributes. For example, 42 grantees use registry data to determine vaccine coverage among different segments of the population, and 32 grantees track VFC participant eligibility by using their registries. CDC is collecting data to monitor projects' use of registries and registry data to support each of these program attributes beginning with the CY 2001 IRAR. The findings in this report are subject to at least three limitations. First, because the annual report relied on self-reported information, bias in reporting could have occurred. However, on-site verification through record reviews and observation of registry operations during the 20 site visits performed in 2000 indicated that 98.3% of the answers provided by those sites in response to the previous year's annual report were accurate. Second, because only information from these 51 immunization grantees was included in the analyses, any immunization registry development performed by other entities (e.g., city immunization grantees, hospitals, local health departments, and managed care plans) would not be included in these findings. This could result in an underestimation of the degree of registry development in the United States. Finally, the CY 2000 IRAR did not collect information about the completeness or accuracy of immunization data recorded in a registry. Tools are under development at CDC to assist with registry data quality assessment. Immunization registries continue to develop to improve vaccination coverage and reach the 2010 national health objective of 95% participation for children aged <6 years. Additional information about immunization registries is available from CDC at http://www.cdc.gov/nip/registry; by telephone at 800-799-7062; or by e-mail at siisclear@cdc.gov. References
Table 1 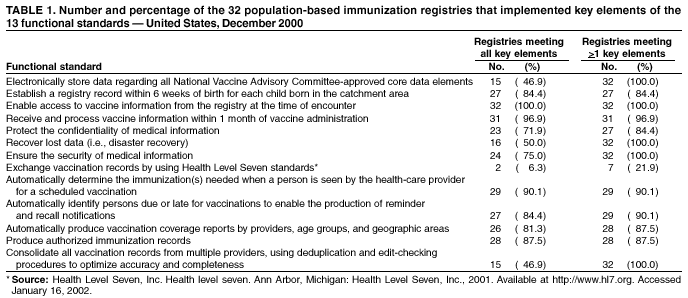 Return to top. Figure 1 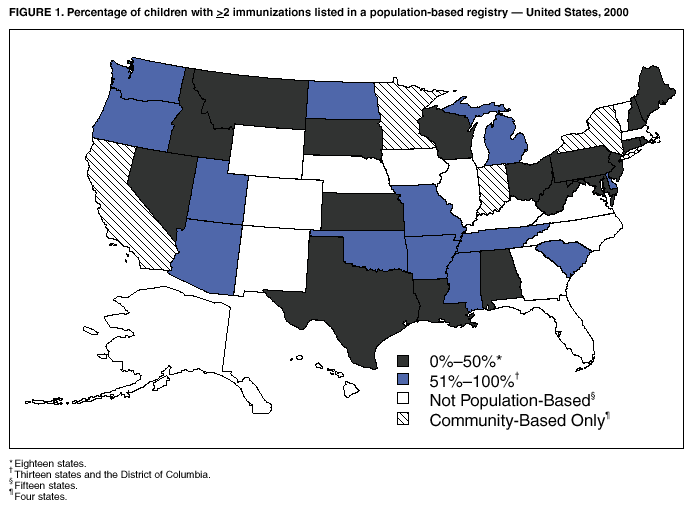 Return to top. Figure 2 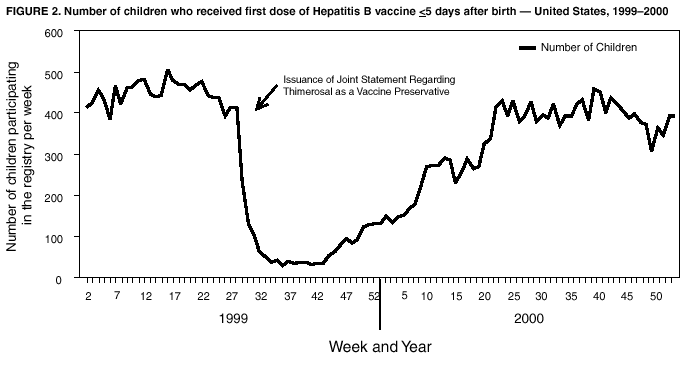 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/24/2002 |
|||||||||
This page last reviewed 1/24/2002
|