 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Update: Influenza Activity -- United States, 1996-97 SeasonIn collaboration with the World Health Organization (WHO), its collaborating laboratories, and state and local health departments, CDC conducts surveillance to monitor influenza activity and to detect antigenic changes in the circulating strains of influenza viruses. This report summarizes influenza surveillance in the United States from September through early November 1996, which indicates that influenza activity is at typical levels for this time of year. From September 4 through November 9, influenza A virus isolates were reported from 10 states (Alaska, California, Colorado, Iowa, Maryland, Montana, New York, North Carolina, Washington, and Wisconsin), and influenza B isolates were reported from seven states (Alaska, Illinois, Kentucky, Missouri, Ohio, Texas, and Wisconsin) (Figure_1). Most isolates were associated with sporadic cases. Of the five influenza type A isolates confirmed at CDC, all were identified as influenza type A(H3N2) and, when further characterized, were closely related to the influenza type A(H3N2) strain included in the 1996-97 influenza vaccine. Of the seven states reporting influenza B, Alaska and Illinois reported isolates obtained from patients who probably became infected while traveling outside the United States (Hong Kong and China, respectively). For the week ending November 9, most state and territorial epidemiologists reported no influenza activity or sporadic * activity; Alaska and Montana reported regional activity. Reported by: Participating state and territorial epidemiologists and state public health laboratory directors. World Health Organization collaborating laboratories. Epidemiology Div, Public Health Laboratory Svcs Communicable Diseases Surveillance Center, United Kingdom. Influenza Br and WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: Levels of activity described in this report are typical for September and October. Although the timing and intensity of influenza activity vary by season, sporadic influenza activity can begin in September, and isolated outbreaks can occur during October and November; widespread influenza activity usually does not begin before December. Although the optimal time for vaccination programs is October through mid-November, health-care providers should continue to offer vaccine to high-risk persons after mid-November and even after influenza activity has been documented in a community. Influenza vaccine contains influenza type A(H1N1), type A(H3N2), and type B strains representing the influenza virus strains that are expected to circulate during the 1996-97 influenza season. The 1996-97 vaccine contains A/Texas/36/91-like (H1N1), A/Wuhan/359/95-like (H3N2), and B/Beijing/184/93-like antigens. For both A/Wuhan/359/95-like and B/Beijing/184-like antigens, U.S. manufacturers used the antigenically equivalent strains A/Nanchang/933/95(H3N2) and B/Harbin/07/94 because of their growth properties. When influenza vaccine is administered after local outbreaks of influenza type A have been reported, short-term prophylaxis with amantadine or rimantadine can be considered. These drugs can be used as treatment or prophylaxis for influenza type A infection, but they are not effective against influenza type B. Because early virologic surveillance has indicated circulation of influenza type A and type B viruses, use of viral culture and rapid antigen-detection testing throughout the season is particularly important (1). Throughout the influenza season, surveillance data collected by CDC will be updated weekly and made available through the CDC voice information system (telephone ({404} 332-4551) and fax information system ({404} 332-4565 and requesting document number 361100). Information about local influenza activity is available from county and state health departments. Reference
Levels of activity are 1) no activity; 2) sporadic -- sporadically occurring influenza-like illness (ILI) or culture-confirmed influenza with no outbreaks detected; 3) regional -- outbreaks of ILI or culture-confirmed influenza in counties with a combined population of less than 50% of the state's total population; and 4) widespread -- outbreaks of ILI or culture-confirmed influenza in counties with a combined population of greater than or equal to 50% of the state's total population. Figure_1 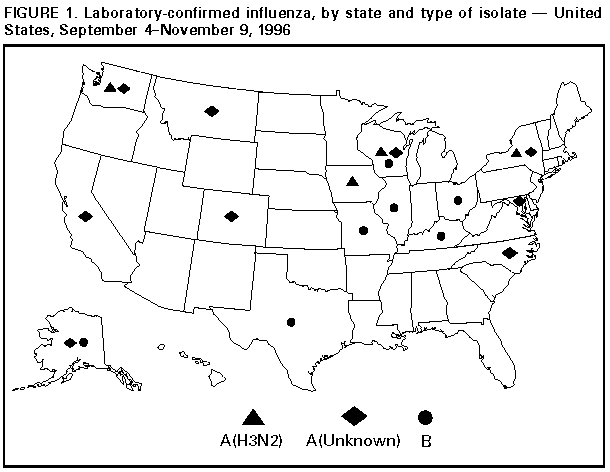 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|