 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Lyme Disease -- United States, 1993In 1982, CDC initiated surveillance for Lyme disease (LD), and in 1990, the Council of State and Territorial Epidemiologists adopted a resolution making LD a nationally notifiable disease. This report summarizes surveillance data for LD in the United States during 1993. LD is defined as the presence of an erythema migrans rash or at least one objective sign of musculoskeletal, neurologic, or cardiovascular disease and laboratory confirmation of infection (1). In 1993, 8185 cases of LD were reported to CDC by 44 state health departments, 1492 (15%) fewer cases than were reported in 1992 (9677) (Figure_1). Most cases were reported from the northeastern, mid-Atlantic, north-central, and Pacific coastal regions (Figure_2). Six states (Alaska, Arizona, Colorado, Mississippi, Montana, and South Dakota) reported no LD cases. The overall incidence rate was 3.3 per 100,000 population. Eight states in established LD-endemic northeastern and upper north-central regions reported rates of more than 3.3 per 100,000 (Connecticut, 41.3; Rhode Island, 27.3; Delaware, 21.0; New York, 15.5; New Jersey, 10.1; Pennsylvania, 8.9; Wisconsin, 8.2; and Maryland, 3.8); these states accounted for 6962 (85%) of the cases reported nationally. Of the total cases, 6132 (75%) were reported from 81 counties that had at least five cases and had rates of at least 10 per 100,000 population. Most (83%) of the decrease in 1993 resulted from reductions in the numbers of case reports from four states in which LD is endemic (California, Connecticut, New York, and Wisconsin). New York, which reported 34% of the U.S. cases in 1993, accounted for 41% of the decrease (609 cases), and Connecticut accounted for 27% of the decrease (410 cases). Thirteen states reported small increases in the number of cases. New Jersey had the largest increase (786 cases, compared with 681 in 1992). The age distribution of persons reported with LD was bimodal, with peaks occurring for children aged 5-14 years (1098 cases) and adults aged 30-49 years (2298 cases). Males (51%) and females were nearly equally affected. Reported by: State health departments. Bacterial Zoonoses Br, Div of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: LD, the most commonly reported vectorborne infectious disease in the United States (2), is caused by the spirochete Borrelia burgdorferi and is transmitted by the bite of an infected Ixodes tick. In the northeastern and upper north-central regions of the United States, the principal tick vector is Ixodes scapularis (black-legged tick), and in Pacific coast states, the principal vector is Ixodes pacificus (western black-legged tick). LD risks are geographically limited; rates vary substantially by town or other geopolitical area within counties (3,4), and the distribution of vector ticks varies greatly, even within individual residential properties (5). LD can be prevented by avoiding contact with the tick vector or by applying insect repellents and acaricides as directed, wearing long pants and long-sleeved shirts, tucking pants into socks, checking regularly for ticks, and promptly removing attached ticks. The decrease in reported cases in 1993 may reflect a combination of three factors: decreased reporting by physicians, decreased case detection (6), and a true decrease in the number of cases. In Connecticut and New York, vector surveillance data suggest that I. scapularis population densities were lower in 1993 than in previous years. The decrease in New York also may be attributed to limitations in staffing and decreased reporting by physicians (D. White, Bureau of Communicable Diseases, New York State Department of Health, personal communication, 1994). The increase in New Jersey was attributed to an increase in reported cases from Hunterdon County as a result of improved reporting by physicians and a true increase in disease incidence (CDC, unpublished data, 1993). The actual incidence of LD in the United States is unknown, and estimates are subject to the influences of underreporting, misclassification, and overdiagnosis. Accurate surveillance data are needed to target populations for LD prevention strategies (e.g., vaccination). In 1993, two U.S. manufacturers received Food and Drug Administration approval to conduct field trials of LD vaccines in humans. One manufacturer is conducting Phase III efficacy trials involving approximately 10,000 participants from endemic areas of the north central, mid-Atlantic, and New England states. The second manufacturer is conducting Phase II safety and immunogenicity trials involving approximately 400 persons residing in New England. Results of Phase I trials conducted in the United States have been published (7), and preliminary results of Phase II safety and efficacy trials (8,9) suggest the vaccine is safe and immunogenic. Both candidate vaccines use a recombinant outer-surface protein as the immunogen. The candidate vaccines stimulate production of antibodies that target B. burgdorferi in the midguts of infected ticks while they extract blood from a vaccinated animal (10). Reliable identification of risks is required for targeting individually applied interventions for LD. LD surveillance data will be needed to determine the effectiveness of control and prevention efforts. References
Figure_1 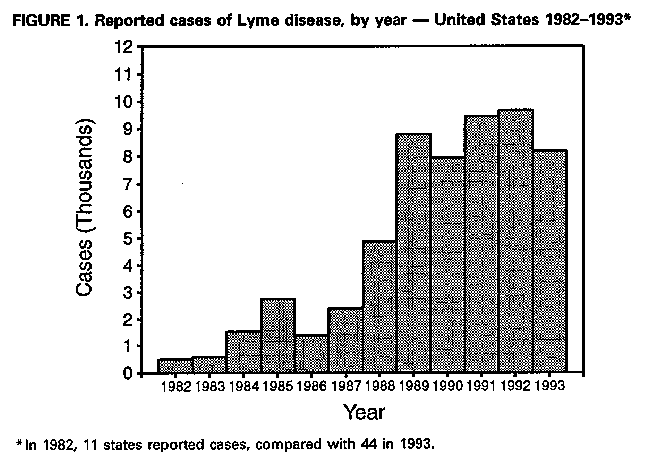 Return to top. Figure_2 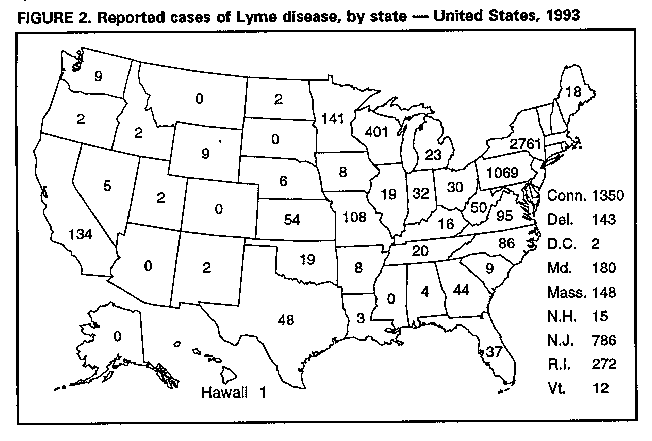 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|