 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Rubella and Congenital Rubella Syndrome -- United States, 1994-1997Indigenous rubella and congenital rubella syndrome (CRS) have been targeted for elimination in the United States by the year 2000 (1). Progress toward reaching this goal is monitored through the National Notifiable Diseases Surveillance System and the National Congenital Rubella Syndrome Registry. From 1969 through 1989, the numbers of annual reported cases decreased 99.6% for rubella and 97.4% for CRS (Figure_1). Following a slight resurgence during 1990-1991, the number of reported rubella cases reached record lows during 1992-1996 (annual average: 183 reported cases). This report summarizes the characteristics of rubella and CRS cases and outbreaks reported in the United States from 1994 through 1996 * and provisional data as of April 18, 1997. The findings indicate sustained low incidence of rubella and CRS since 1992 and possible interruption of transmission of rubella virus in late 1996. Rubella During 1994-1996, a total of 32 states, the District of Columbia, and New York City reported 567 rubella cases; 22 sites reported one to five cases, seven reported six to 19 cases, and five reported greater than or equal to 20 cases; these five sites accounted for 75% of all rubella cases (Figure_2). Symptom onset for reported confirmed cases peaked during February 1994, June 1995, and April 1996, reflecting large outbreaks in Massachusetts, Connecticut, and North Carolina (range: 36-128 cases). Based on provisional data as of April 18, 1997, symptom onset for the last case in 1996 was November 6 and for the first case in 1997 was January 5, representing approximately three incubation periods with no reported rubella cases. Of the 561 (98.9%) case-patients for whom age was known, 474 (84.5%) were aged greater than or equal to 15 years, and 422 (75.2%) were aged 15-44 years. Of the 563 (99.3%) case-patients for whom sex was reported, 313 (55.6%) were male. Of the 560 case-patients for whom both age and sex were known, 171 (30.5%) were women of childbearing age (15-44 years); of these, five were pregnant at the time of rash onset. Race was known for 477 (84.1%) case-patients; of these, 379 (79.4%) were white, 30 (6.3%) were Asian/Pacific Islander, and 20 (4.2%) were black. Of the 492 (86.8%) case-patients for whom ethnicity was known, 266 (54.1%) were Hispanic; the percentage of cases among Hispanics increased from 19.0% in 1991 to 68.1% in 1996 (2). Since 1994, six outbreaks have involved five or more cases. The three largest outbreaks (Massachusetts, Connecticut, and North Carolina) accounted for 249 (44.1%) of the cases reported during 1994-1996; most cases occurred among adults for whom history of vaccination was unavailable. In each of these outbreaks, transmission occurred in multiple settings (e.g., workplaces, homeless shelters, a substance-abuse treatment center, a publicly supported hospital, and a county jail). In smaller outbreaks, reported cases occurred primarily among adults in a workplace, a county jail, and a college. Of the the 500 (88.2%) reported cases for which adequate information was available, 481 (96.2%) were confirmed, 17 (3.4%) were probable, and two (0.4%) were suspected. For the 169 (35.1%) confirmed cases for which method of confirmation was reported, 142 (84.0%) were laboratory confirmed, and 27 (16.0%) were confirmed by epidemiologic linkage to a laboratory-confirmed case. Of the 505 (89.1%) cases with known importation status, 471 (93.3%) were indigenously acquired, 32 (6.3%) were internationally imported, and two (0.4%) were imported from another state. Of the internationally imported cases, country of exposure was reported for 15 (46.9%) and included Mexico (five cases); Japan (three); Kenya (two); and Colombia, England, Germany, Korea, and Switzerland (one each). Congenital Rubella Syndrome A total of 12 infants with laboratory-confirmed CRS were born during 1994-1996. Nine states reported seven indigenously acquired cases, four imported cases, and one case with unknown importation status. The maternal exposures for the four imported cases occurred in Mexico (two cases), Sri Lanka (one), and Dominican Republic (one) -- countries that do not routinely provide rubella vaccination. Of the seven infants with indigenously acquired cases, four were born to women of Hispanic ethnicity; of the three with documented sources of exposure, two were outbreak-related and one had contact with an infected relative. Of 10 mothers for whom information was available, seven had one or more missed opportunities for vaccination. Reported by: State and territorial epidemiologists. Child Vaccine Preventable Diseases Br, Epidemiology and Surveillance Div, National Immunization Program, CDC. Editorial NoteEditorial Note: Since 1994, incidences of rubella and CRS have been sustained at record low levels. Indigenous transmission of rubella appears to have been interrupted in the United States at least once; however, infection can be reintroduced through importation from neighboring countries that do not routinely promote rubella vaccination. Since 1994, most rubella and CRS cases have been associated with outbreaks among unvaccinated adults, and three fourths of reported rubella cases occurred among persons aged 15-44 years, a substantial increase from 1966-1968, when only 23% of reported rubella infections occurred among persons aged greater than or equal to 15 years (3). In recent years, outbreaks of rubella have occurred primarily in settings where young adults congregate, and the risk has been highest among persons in specific racial/ethnic groups (e.g., Hispanics) who often are unvaccinated and who may be exposed to persons traveling from areas where rubella vaccination is not routine. The increasing proportion of cases accounted for by persons of Hispanic ethnicity suggests a potentially susceptible group of persons to whom vaccination efforts should be directed. In two of the larger outbreaks, 98% of cases occurred among persons of Hispanic ethnicity. Hispanics and persons who are natives of countries without rubella vaccination programs should be considered susceptible to rubella unless they have documentation of vaccination or serologic evidence of immunity. The changing epidemiologic pattern of rubella underscores the importance of ongoing collection and analysis of information on reported rubella and CRS cases, including demographics, vaccination history, source of exposure (i.e., indigenous or imported), relation to outbreaks, and mode of transmission. Such analysis is important for effectively targeting vaccination activities, evaluating the effectiveness of rubella and CRS prevention programs, and designing more efficient prevention strategies. The effectiveness of efforts to control and prevent rubella in the United States is reflected by possible interruption of transmission of rubella during November-December 1996, the dramatic decline in reported cases when compared with the prevaccine era, and the low annual average number of cases since 1991. However, elimination of indigenously acquired rubella and CRS in the United States will require 1) maintenance of high vaccination levels in preschool- and school-aged children and young adults, 2) intensification of diagnosis of and surveillance for rubella and CRS, and 3) prompt control of outbreaks (4-6). The shift in the increasing proportion of cases accounted for by persons aged 15-44 years indicates that vaccination programs targeting school-aged children have been successful in preventing rubella in that age group but that vaccination activities also should include adolescents and adults. Because more than half of CRS cases in recent years have resulted from missed opportunities for vaccination (7), health-care providers should screen reproductive-aged women for rubella immunity (e.g., during prenatal screenings and premarital health-care visits) and vaccinate when appropriate (e.g., postpartum). Elimination of indigenous transmission of rubella in the United States also will require collaboration with other countries to develop and implement national rubella vaccination policies. References
* Reports for 1996 are provisional. Figure_1 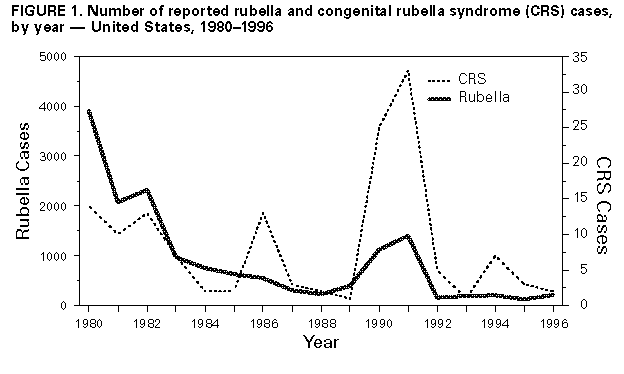 Return to top. Figure_2 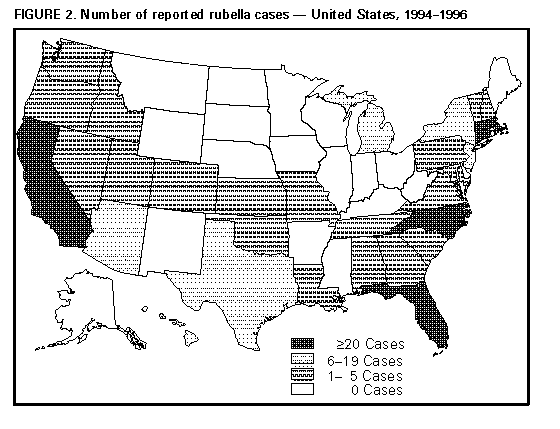 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|