 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Update: Influenza Activity --- United States, 2003--04 SeasonInfluenza began circulating in the United States unusually early this season, and influenza activity nationwide is expected to increase. Cases of severe disease, including deaths, have been reported in children. This report summarizes influenza activity in the United States during the weeks ending October 4--December 6, 2003*. During the week ending December 6, influenza activity was reported to CDC as widespread in 24 states (Figure). The early season and the unusually high and persistent demand for vaccine have resulted in a decreasing supply of trivalent inactivated vaccine. Emphasis should be placed on vaccinating persons at high risk for complications from influenza, including healthy children aged 6--23 months. Healthy persons aged 5--49 years who wish to receive vaccine should consider being vaccinated with the intranasally administered live, attenuated influenza vaccine (LAIV), a substantial supply of which remains available. National SurveillanceCDC conducts national influenza surveillance by monitoring 1) viruses through a system of approximately 120 World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) laboratories, 2) visits for influenza-like illness (ILI)† through the U.S. Influenza Sentinel Providers Surveillance Network, 3) the percentage of U.S. deaths attributable to pneumonia and influenza (P&I) reported through the 122 Cities Mortality Reporting System, and 4) estimated levels of influenza activity reported to CDC by state and territorial epidemiologists. CDC also receives reports from clinicians and local health officials on influenza outbreaks and cases nationwide. Influenza Virus SurveillanceFor the weeks ending October 4--December 6, WHO and NREVSS collaborating laboratories in the United States tested 24,906 respiratory specimens for influenza viruses; 6,751 (27.1%) were positive. During the same period, the weekly percentages of respiratory specimens testing positive for influenza viruses increased from 1.4% to 37.1%. During the 2000--01, 2001--02, and 2002--03 influenza seasons, the peak percentages of specimens testing positive for influenza ranged from 23.2% to 26.4%. During the 1999--00 influenza season, when influenza A (H3N2) viruses predominated, the peak weekly percentage of specimens testing positive was 30.9% (1; CDC, unpublished data, 2003). Of the 6,751 positive isolates, 6,716 (99.5%) were influenza A viruses, and 35 (0.5%) were influenza B viruses. Of the 6,716 influenza A viruses, 1,255 (18.7%) have been subtyped; 1,254 (99.9%) were influenza A (H3N2) viruses, and one (0.1%) was an influenza A (H1) virus. As of December 6, a total of 47 states and all nine surveillance regions had reported laboratory-confirmed influenza. CDC has characterized antigenically 215 influenza viruses that were collected and submitted by U.S. laboratories since October 1. Of these, 212 were influenza A (H3N2) viruses, and one was an influenza A (H1) virus. Of the 212 influenza A (H3N2) viruses, 54 (25%) were similar antigenically to the vaccine strain A/Panama/2007/99 (H3N2), which is contained in this season's vaccine, whereas 158 (75%) were similar antigenically to A/Fujian/411/2002, a drift variant of A/Panama/2007/99. ILI SurveillanceDuring the weeks ending October 4--December 6, the weekly percentages of patient visits§ to approximately 1,000 sentinel providers nationwide for ILI increased from 0.9% to 5.1%, which is above the national baseline¶ of 2.5%. During the 2000--01, 2001--02, and 2002--03 influenza seasons, the peak weekly percentages of patient visits for ILI ranged from 3.3% to 4.4%. During the 1999--00 season, the peak weekly percentage for patient visits for ILI was 7.1% (1; CDC, unpublished data, 2003). P&I Mortality SurveillanceDuring the week ending December 6, P&I accounted for 7.0% of all deaths reported through the 122 Cities Mortality Reporting System. The epidemic threshold** for that week was 7.6%. Since the week ending October 4, the weekly percentage of P&I deaths has been below the epidemic threshold. The percentage of P&I deaths exceeded the epidemic threshold for zero weeks during the 2002--03 influenza season, for 9 weeks during the 2001--02 season, and for 10 weeks during the 2000--01 influenza season. During the 1999--00 influenza season, the percentage of P&I deaths exceeded the epidemic threshold for 15 weeks (1; CDC, unpublished data, 2003). Activity Reported by State and Territorial EpidemiologistsDuring the week ending December 6, influenza activity†† was reported as widespread in 24 states (Alaska, Arizona, Arkansas, Colorado, Idaho, Indiana, Iowa, Mississippi, Missouri, Montana, Nebraska, Nevada, New Mexico, North Carolina, Oklahoma, Oregon, Pennsylvania, Rhode Island, Tennessee, Texas, Utah, Virginia, Washington, and Wyoming), regional in 15 states (Alabama, California, Connecticut, Florida, Georgia, Illinois, Kansas, Kentucky, Maryland, Minnesota, New York, North Dakota, Ohio, South Carolina, and West Virginia) and New York City, and local in six states (Louisiana, Massachusetts, Michigan, New Jersey, South Dakota, and Vermont) and the District of Columbia. Sporadic influenza activity was reported in five states (Delaware, Hawaii, Maine, New Hampshire, and Wisconsin) and Guam. Reports of Severe Illness and DeathsPediatric cases. CDC has received reports of severe complications of influenza occurring in young infants, school-age children, and adolescents. Complications have included encephalopathy, seizures, dehydration with severe hypotension, respiratory failure requiring mechanical ventilation, and secondary bacterial pneumonia, including necrotizing pneumonia with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Three deaths (an infant aged 20 months with underlying reactive airways disease, a previously healthy infant aged 22 months, and a previously healthy child aged 16 years) have been associated with secondary pneumonia caused by CA-MRSA. Other influenza-related deaths not related to CA-MRSA in children have occurred. Fatal cases reported to CDC are being investigated by local and state health authorities. Laboratory testing has confirmed influenza A virus infection in these fatal cases; antigenic characterization is pending. The vaccination status of the majority of the deceased children has not been determined. Pregnant women. In Texas, 88 pregnant women had laboratory-confirmed influenza A infections. Symptoms included fever, cough, and profound sinus tachycardia (i.e., 150--170 beats per minute) that resolved subsequently. One patient required intensive care for bilateral pneumonia and myocarditis. Of the 88 patients, two (2.3%) had been vaccinated 2 and 10 days before admission, respectively. No influenza-associated maternal deaths occurred; one case of fetal loss occurred but was not attributed to maternal influenza infection. The majority of the 88 cases were associated with influenza A infection; however, influenza B viruses also were detected. Reported by: S Harper, MD, T Uyeki, MD, E Murray, MSPH, L Brammer, MPH, J Wright, DVM, K Fukuda, MD, N Cox, PhD, Div of Viral and Rickettsial Diseases; C McDonald, Div of Healthcare Quality Promotion, National Center for Infectious Diseases; M Wharton, MD, Epidemiology and Surveillance Div, National Immunization Program, CDC. Editorial Note:Influenza seasons can vary substantially in terms of timing and pattern of onset, peaking, decline, and overall severity. In the United States, the 2003--04 influenza season began unusually early, with community activity first reported in early October, followed by continued spread of influenza activity during the weeks ending October 4--December 6. National activity levels have not yet peaked, and neither the duration of activity nor the season's eventual magnitude is known. As of December 6, influenza A (H3N2) viruses predominated in the United States, but different influenza viruses might predominate later in the season. Influenza seasons dominated by A (H3N2) viruses (e.g., those in 1996--97, 1997--98, and 1998--99) typically are associated with high levels of severe illness and deaths (3). No evidence exists to indicate that the A/Fujian-like viruses in circulation are more virulent than other influenza A (H3N2) viruses. However, reports of severe pediatric illnesses and deaths underscore the severe consequences that influenza infections can cause in children (4). Cases of sudden death associated with influenza in previously healthy children also were reported in the United States during the 2002--03 season (4; CDC unpublished data, 2003). Although the pathophysiology of sudden deaths associated with influenza in children is unknown, atypical symptoms (e.g., abdominal pain, absence of fever, and mild respiratory symptoms) have been reported. Encephalopathy is another severe and potentially under-recognized complication of influenza in children (5). One case so far this season has resulted in the death of a patient (CDC, unpublished data, 2003). Patients might report high fevers, seizures, headaches, abnormal mental status, and/or confusion and do not always exhibit classic influenza symptoms. Cases have been reported among young children and school-aged children, including adolescents. Suspected cases should be reported to CDC at telephone, 404-639-0277 or 404-639-2893; fax, 404-639-3866; or e-mail, tmu0@cdc.gov or nib9@cdc.gov. Although secondary bacterial pneumonia is a common complication of influenza infection, S. aureus typically occurs in a minority of such cases. Clinical and laboratory features of S. aureus pneumonia are similar to other types of community-acquired pneumonia (6,7). Clinicians should be aware that CA-MRSA can be a cause of community-acquired pneumonia. Treatment for pneumonia after influenza infection should be guided by bacterial culture results when possible. Aspirin and other salicylate-containing medications should not be administered to children with fever and respiratory illness (1). Pregnant women are at higher risk than nonpregnant women for having complications secondary to influenza. Pregnant women who will be in their second or third trimester during influenza season should be vaccinated against influenza (8). So far this season, influenza A/Fujian/411/2002-like viruses are predominating in the United States. This strain differs from the influenza A (H3N2) virus contained in the 2003--04 vaccine (i.e., A/Panama/2007/99). The A/Fujian-like viruses are antigenic drift variants of the A/Panama strain and were detected by global surveillance early this year but too late for inclusion in the current influenza vaccine. Hemagglutination inhibition testing using postinfection ferret sera indicates that antibodies to the A/Panama vaccine virus cross-react with A/Fujian-like viruses; therefore, current influenza vaccines should provide some protection against A/Fujian-like viruses. However, the level of protection remains uncertain until vaccine effectiveness studies are completed. The vaccine also contains A/New Caledonia/20/99 (H1N1)-like and B/Hong Kong/330/2001-like viruses and should protect persons who are vaccinated against these viruses if they circulate more widely later in the season. Approximately 83.4 million doses of influenza vaccine, including inactivated influenza vaccine made by two manufacturers and LAIV made by a third manufacturer, were produced for the 2003--04 influenza season. All doses of trivalent inactivated vaccine appear to have been sold by the manufacturers and their major distributors. Trivalent inactivated vaccine remains available from physicians' offices and in other settings. As of December 9, a total of 3.9 million doses of LAIV were available from the manufacturer (Wyeth Pharmaceuticals, Collegeville, Pennsylvania, telephone 800-358-7443). To ascertain the availability of influenza vaccine, CDC conducted a survey of state and urban area immunization programs. As of December 3, a total of 28 states had redistributed influenza vaccine from health-care providers and public immunization clinics that had excess supplies to those that needed vaccine. In addition, 34 states had influenza vaccine inventory that had not been distributed. However, in an average year, <10% of influenza vaccine is purchased by state health departments. Influenza antiviral medications are available for use in adults and children. Four prescription antiviral medications (i.e., amantadine, rimantadine, oseltamivir, and zanamivir) are approved for treatment of influenza A virus infections. Oseltamivir and zanamivir also are approved for treatment of influenza B. The costs, routes of administration, adverse effects, contraindications, approved ages, and potential for antiviral resistance differ among the four drugs. When administered within 48 hours of symptom onset, antiviral treatment of influenza can reduce the duration of illness by approximately 1 day in healthy adults (9). Data on the use of any of the four antiviral agents during pregnancy are not available. Amantadine, rimantadine, and oseltamivir also are approved for chemoprophylaxis of influenza A virus infections and can be used for control of institutional influenza outbreaks. When used for chemoprophylaxis, antivirals can be approximately 70%--90% effective in preventing illness in healthy adults (9,10). To obtain information about approved age groups, dosing, and adverse effects, clinicians should consult antiviral drug package inserts (available from the Food and Drug Administration at http://www.fda.gov/cder/drug/antivirals/influenza/default.htm#drugs). CDC has published recommendations for prevention and control of influenza (available at http://www.cdc.gov/mmwr/PDF/rr/rr5208.pdf). Supplemental recommendations have been released for the 2003--04 influenza season (Box). Influenza surveillance reports for the United States are published weekly during October--May and are available from CDC at http://www.cdc.gov/flu or through CDC's voice (telephone, 888-232-3228) and fax (telephone, 888-232-3299, document number 361100) information systems. Acknowledgments This report is based on data contributed by A Tulu, K Hankins, Dallas County Health and Human Svcs Office; G Wendell, J Sheffield, Parkland Memorial Hospital, Dallas; J Siegel, Children's Medical Center, Dallas; N Pascoe, S Avashia, Texas Dept of Health. K Gershman, Colorado State Dept of Public Health and Environment. Participating state and territorial epidemiologists and state public health laboratory directors. WHO collaborating laboratories. National Respiratory and Enteric Virus Surveillance System collaborating laboratories, U.S. Influenza Sentinel Provider Surveillance System. Div of Public Health Surveillance and Informatics, Epidemiology Program Office; DJ O'Mara, Immunization Svcs Div, National Immunization Program, CDC. References
* Data reported as of December 5. † Temperature of >100.0º F (37.8º C) and cough and/or sore throat in the absence of a known cause other than influenza. § National and regional percentages of patient visits for ILI are weighted on the basis of state population. ¶ Calculated as the mean percentage of visits for ILI during noninfluenza weeks, plus two standard deviations. Wide variability in regional data precludes calculating region-specific baselines and makes it inappropriate to apply the national baseline to regional data. ** The expected baseline proportion of P&I deaths reported by the 122 Cities Mortality Reporting System is projected by using a robust regression procedure that applies a periodic regression model to the observed percentage of deaths from P&I during the previous 5 years; the epidemic threshold is 1.645 standard deviations above the seasonal baseline percentage (2). †† Levels of activity are 1) no activity, 2) sporadic---small numbers of laboratory-confirmed influenza cases or a single influenza outbreak reported but no increase in cases of ILI, 3) local---outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in a single region of a state, 4) regional---outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in at least two but less than half the regions of a state, and 5) widespread---outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in at least half the regions of a state.
Figure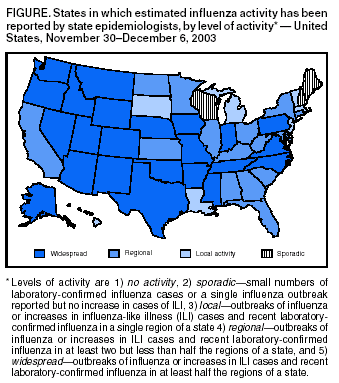 Return to top. Box  Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/11/2003 |
|||||||||
This page last reviewed 12/11/2003
|