 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Travel-Associated Dengue Infections --- United States, 2001--2004Please note: An erratum has been published for this article. To view the erratum, please click here. Dengue is a mosquito-transmitted, acute viral disease caused by any of the four dengue virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4). Dengue is endemic in most tropical and subtropical areas of the world and has occurred in U.S. residents returning from travel to such areas (1--3). CDC maintains a laboratory-based passive surveillance system for travel-associated dengue among U.S. residents*. The system relies on voluntary reports submitted to state health departments by clinicians; patient specimens are then forwarded to CDC for diagnostic testing. This report summarizes information about travel-associated dengue cases among U.S. residents during 2001--2004. The risk for dengue infection among travelers can be reduced by use of repellents and by avoiding exposure to mosquitoes. Serum samples from 366 patients who had suspected dengue on the basis of clinical presentation and onset of symptoms (4) during 2001--2004 were submitted to CDC from 37 states and the District of Columbia (107 suspected infections in 2001, 74 suspected infections in 2002, 95 suspected infections in 2003, and 90 suspected infections in 2004). Of the 366 suspected infections with sera submitted for testing, 77 (21%) were laboratory-diagnosed as acute dengue infections. Of these 77 patients, 67 (87%) had dengue infection diagnosed by elevated anti-dengue IgM antibodies, and 10 patients (13%) had infection diagnosed after isolation of dengue virus (DEN-1, DEN-2, or DEN-3) from their serum (Table). Of the 77 acute infections, eight (10%) were diagnosed as primary infections, and 12 (16%) were secondary infections. For the remaining 57 cases (74%), whether the infection was the patient's first dengue infection or a subsequent infection could not be determined either because a convalescent sample (collected more than 5 days after symptom onset) was not submitted, or both samples were collected during the convalescent phase of infection. Among the 366 suspect cases, dengue testing was negative in 183 patients (50%). A total of 22 patients (6%) had elevated IgG titers, suggesting that a flavivirus infection or vaccination (e.g., yellow fever) had occurred in the past but that infection was not the cause of the acute symptoms. For 88 patients (24%), the result of dengue testing was indeterminate because a convalescent sample for serologic testing was unavailable. Of the 77 patients with laboratory-diagnosed dengue infections, 41 (53%) were female. The median age of the 71 patients for whom age was reported was 38 years (range: 8 months--72 years). Clinical information was available for 56 patients (73%). The most commonly reported symptoms were fever (54 patients [96%]), headache (36 [64%]), myalgias (32 [57%]), chills (19 [34%]), and rash (20 [36%]). Fourteen patients (25%) had at least one hemorrhagic symptom (e.g., petechiae, purpura, hemoptysis, hematemesis, hematuria, or epistaxis), and nine (16%) had elevated liver transaminases. Because of incomplete reporting, whether any of the laboratory-diagnosed cases met the clinical criteria for dengue hemorrhagic fever (DHF) could not be determined; however, 15 patients (27%) required hospitalization, including one who died. The fatal case occurred in an adult in otherwise good health who had recently returned from a month-long visit to Mexico. Travel destinations were available for 66 patients (86%); 20 patients (30%) reported recent travel to a Caribbean island during the 2 weeks before illness onset, 14 (21%) to Pacific islands, 11 (17%) to Asia, 10 (15%) to Central America, 10 (15%) to South America, and one (2%) to Africa. Ten patients acquired their dengue infections during travel to areas of the United States in which dengue is endemic (Puerto Rico [six] and U.S. Virgin Islands [two]) or to a U.S. location where an outbreak was occurring (Hawaii during May 27, 2001--January 30, 2002 [two]) (5). Reported by: ME Beatty, MD, V Vorndam, PhD, EA Hunsperger, PhD, JL Muñoz, PhD, GG Clark, PhD, Div of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, CDC. Editorial Note:Dengue is transmitted to humans by Aedes mosquitoes. Prevention of dengue among traveling U.S. residents is possible by avoiding exposure to mosquitoes. Preventing dengue infection not only benefits the traveler but also prevents establishment of autochthonous dengue transmission in areas of the United States in which a competent vector is abundant but dengue virus is absent. During an outbreak that began on May 27, 2001, a total of 122 Hawaii residents tested positive for recent dengue infection after establishment of autochthonous dengue transmission (5). This outbreak resulted in 45 more cases of laboratory-diagnosed dengue infections than were reported among U.S. travelers from the remaining 49 states for the period 2001--2004. Autochthonous transmission of dengue was also documented in Texas in 1999, when 66 patients with laboratory-diagnosed dengue were identified (6). The incubation period for dengue has a range of 3--14 days (in the majority of cases, 4--7 days). Dengue virus infection can be asymptomatic or cause illnesses ranging from mild undifferentiated fever to severe disease, including hemorrhagic manifestations and shock (7). DHF is characterized by fever, minor or major bleeding phenomena, thrombocytopenia (<100,000 platelets/µL), and evidence of increased vascular permeability (e.g., hemoconcentration [hematocrit increased by >20% from baseline], pleural or abdominal effusions, or hypoproteinemia) (7). Previous dengue infection increases the risk for DHF in a patient with subsequent dengue infection. Dengue shock syndrome is DHF with signs of circulatory failure, including narrow pulse pressure (<20 mm Hg), hypotension, or shock, and can result in a case-fatality rate of approximately 10% (8). The findings in this report are subject to at least one limitation. These data are likely subject to underreporting because 1) the system is passive (i.e., relies on providers to report infection), 2) dengue reporting is not a nationally notifiable disease in the United States, and 3) reporting is tied to specimen submission (i.e., if testing is completed outside of CDC, this system would not capture the results). Persons traveling to areas in which dengue is endemic should avoid exposure to mosquitoes by using repellents, wearing protective clothing, and remaining in well-screened or air-conditioned areas. No vaccine is available for preventing dengue infection. Health-care providers should consider dengue in the differential diagnoses of illness for all patients who have fever and a history of travel to tropical and subtropical areas within 2 weeks before the onset of symptoms. Supportive measures should be administered, and only acetaminophen is recommended for management of pain and fever. Acetylsalicylic acid (i.e., aspirin) and other nonsteroidal anti-inflammatory agents are contraindicated because of their anticoagulant properties and, in the case of children, because of their association with Reye Syndrome. Patients with dengue should be monitored for signs of DHF, especially hypotension, because prompt fluid therapy reduces morbidity and mortality. Acute-phase (0--5 days after onset of symptoms) and convalescent-phase (6--30 days after onset of symptoms) serum samples obtained for viral isolation and serologic diagnosis, respectively, should be sent through state or territorial health departments to CDC's Dengue Branch, Division of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, 1324 Calle Cañada, San Juan, PR 00920-3860, telephone 787-706-2399, fax 787-706-2496. Serum samples should be accompanied by a summary of clinical and epidemiologic information, including date of disease onset, date of sample collection, and a detailed recent travel history. Additional information for health-care providers regarding dengue case reporting and instructions for specimen shipping are available at http://www.cdc.gov/ncidod/dvbid/dengue/dengue-hcp.htm. Acknowledgment This report is based, in part, on data contributed by state and local health departments. References
* Laboratory-diagnosed dengue in a U.S. resident living in an area without known autochthonous dengue transmission, with travel history in the 14 days before symptom onset to an area of the world with autochthonous dengue transmission. Table 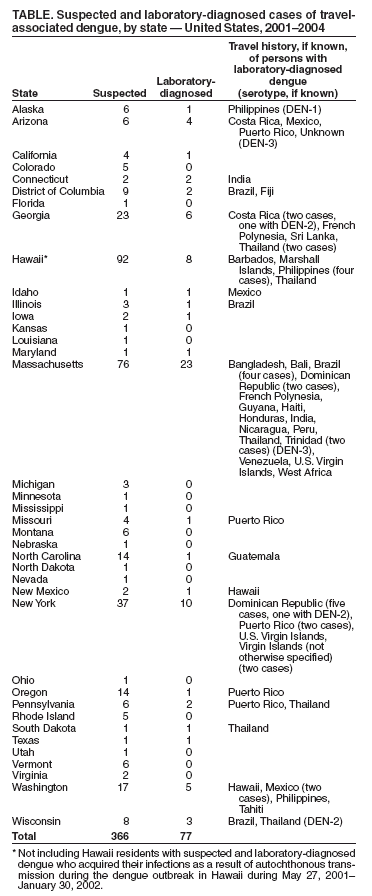 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/9/2005 |
|||||||||
|