|
« Factbook Table of
Contents
9. Research Grants
NHLBI Research Grants by Funding Mechanism: Fiscal Year
2005
| |
Number of Grants |
Total Cost (Dollars in Thousands) |
Percent of Total NHLBI Research Grant Dollars |
| Research Project
Grants (RPGs) |
|
|
|
|
Research Project
Grants (Excluding Small Business RPGs) |
|
|
|
|
Regular Research Grants
(R01) |
|
|
57.51% |
|
Small Research Grants
(R03) |
|
73 |
0.00 |
|
Program Project Grants
(P01) |
|
380,358 |
16.46 |
|
Cooperative Agreements
(U01) |
|
203,546 |
8.81 |
|
Area Grants (R15) |
|
4,201 |
0.18 |
|
Explorative Developmental
Grant (R21) |
|
20,232 |
0.88 |
|
Method to Extend Research in
Time (R37) |
|
30,667 |
1.33 |
|
Subtotal, Research Project
Grants (Excluding Small Business RPGs) |
3,998 |
1,967,810 |
85.17 |
|
Small Business
Research Project Grants |
|
|
|
|
Small Business Technology
Transfer (STTR Phase I) (R41) |
25 |
4,212 |
0.18 |
|
Small Business Technology
Transfer (STTR Phase II) (R42) |
13 |
5,291 |
0.23 |
|
Small Business Innovation
Research (SBIR Phase I) (R43) |
90 |
14,017 |
0.61 |
|
Small Business Innovation
Research (SBIR Phase II) (R44) |
104 |
50,220 |
2.17 |
|
Small Business Innovation
Research (SBIR) Cooperative Agreements (U44) |
--- |
500 |
0.02 |
|
Subtotal, Small Business
Research Project Grants |
232 |
74,240 |
3.19 |
| Subtotal, Research
Project Grants |
4,230 |
2,042,050 |
88.37 |
| Research Center
Grants |
|
|
|
|
Specialized Centers of
Research (SCOR) |
52 |
115,730 |
5.01 |
|
Animal Model and Animal and
Biological Material Resource Grants (P40) |
--- |
125 |
0.01 |
|
Sickle Cell Centers
(U54) |
12 |
24,314 |
1.05 |
|
Center for AIDS Research
(P30) |
--- |
2,815 |
0.12 |
|
Specialized Centers
(Cooperative Agreements) (U54) |
5 |
8,186 |
0.35 |
|
National Swine Research and
Resource Center (U42) |
--- |
325 |
0.01 |
| Subtotal, Research
Center Grants |
69 |
151,495 |
6.56 |
| Research Career
Programs |
|
|
|
|
Mentored Research Development
Award for Minority Faculty (K01) |
45 |
6,088 |
0.26 |
|
Minority Institution Faculty
Mentored Research Scientist Award (K01) |
4 |
588 |
0.03 |
|
Mentored Scientist Development
Award in Research Ethics (K01) |
3 |
355 |
0.02 |
|
Independent Scientist Award
(K02) |
32 |
3,218 |
0.14 |
|
Research Career Award
(K06) |
1 |
34 |
0.00 |
|
Cultural Competence and Health
Disparities Academic Award (K07) |
14 |
1,620 |
0.07 |
|
Clinical Investigator
Scientist Award (K08) |
239 |
30,429 |
1.32 |
|
Career Enhancement Award for
Stem Cell Research (K18) |
3 |
512 |
0.02 |
|
Mentored Patient-Oriented
Research Career Development Award (K23) |
127 |
17,086 |
0.74 |
|
Midcareer Investigator Award
in Patient-Oriented Research (K24) |
32 |
3,929 |
0.17 |
|
Mentored Quantitative Research
Career Development Award (K25) |
17 |
2,206 |
0.10 |
|
Clinical Research Curriculum
Award (K30) |
--- |
4,589 |
0.20 |
|
Career Transition Award
(K22) |
2 |
364 |
0.02 |
| Subtotal, Research
Career Programs |
519 |
71,018 |
3.08 |
| Other Research
Grants |
|
|
|
|
Cooperative Clinical Research
(U10, R10) |
38 |
26,295 |
1.14 |
|
Minority Biomedical Research
Support (S06, S14, R25) |
--- |
2,846 |
0.12 |
|
Other (R09, R13, R18, R24,
R25, T15, U09, U24, UH1) |
98 |
16,554 |
0.72 |
| Subtotal, Other Research
Grants |
136 |
45,695 |
1.98 |
| Total, NHLBI
Research Grants |
4,954 |
$2,310,258 |
100% |
NHLBI Total Research Grants by Category
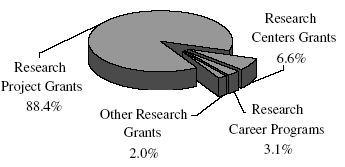
Text-only with data points
Back to Top
NHLBI Research Project Grant,*
Research Centers Grant, and Other Research Grant Obligations: Fiscal Years
1995–2005
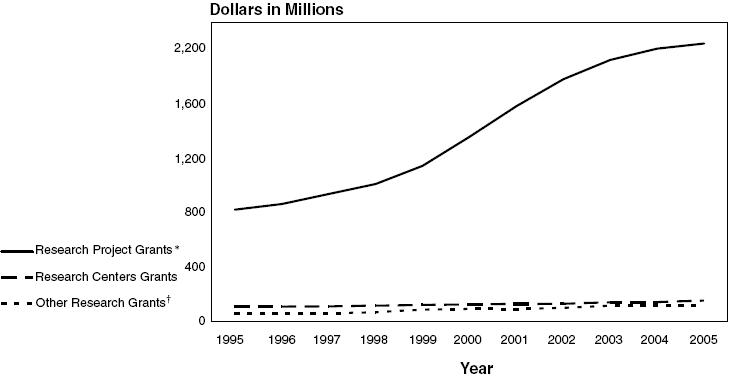
Text-only with data points are represented in the
table below.
NHLBI Research Project Grant,* Research Centers
Grant, and Other Research Grant Obligations: Fiscal Years 1995–2005
| |
Dollars
(Thousands) |
| |
Fiscal
Year |
| |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005 |
| Research |
$819,674 |
$862,027 |
$935,322 |
$1,009,152 |
$1,142,473 |
$1,356,034 |
$1,580,751 |
$1,779,573 |
$1,920,201 |
$2,003,769 |
$2,042,050 |
| Project |
|
|
|
|
|
|
|
|
|
|
|
| Grants* |
|
|
|
|
|
|
|
|
|
|
|
| Research |
106,980 |
106,688 |
108,665 |
114,397 |
119,889 |
123,803 |
127,232 |
128,161 |
138,941 |
140,600 |
151,495 |
| Centers |
|
|
|
|
|
|
|
|
|
|
|
| Grants |
|
|
|
|
|
|
|
|
|
|
|
| Other |
|
|
|
|
|
|
|
|
|
|
|
| Research |
55,974 |
56,692 |
56,993 |
66,234 |
84,219 |
90,666 |
88,958 |
98,460 |
113,172 |
112,785 |
116,713 |
| Grants† |
|
|
|
|
|
|
|
|
|
|
|
| Total |
$982,628 |
$1,025,407‡ |
$1,100,980 |
$1,189,783 |
$1,346,581 |
$1,570,503 |
$1,796,941 |
$2,006,194 |
$2,172,314 |
$2,257,154 |
$2,310,258 |
* Includes R01, R03,
U01, P01, R37, R41, R43, and R44; R29 in 1994–2002; R55 in 1995–1996;
R15 and R42 beginning in 1996; R21 beginning in 1997; and R33 beginning in
2001.
† Includes Research Career Programs; excludes General Research
Support Grants.
‡ Includes Program Evaluation and IMPAC II Assessment
of $4,435,000. |
Back to Top
NHLBI Competing Research Project
Grant Applications*: Fiscal Years 1995–2005
Total Cost Dollars Reviewed and
Awarded
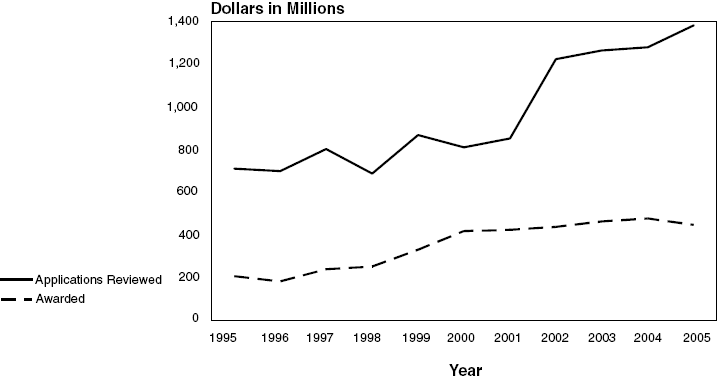
Text-only with data points are represented in the
table below.
NHLBI Competing Research Project Grant Applications:
Fiscal Years 1995–2005
Total Cost Dollars Reviewed and
Awarded
| Dollars
(Millions) |
| Fiscal
Year |
| |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005† |
| Applications Reviewed |
$710.3 |
$699.2 |
$802.1 |
$687.1 |
$867.1 |
$809.8 |
$851.7 |
$1,221.7 |
$1,262.5 |
$1,277.6 |
$1,381.0 |
| Awarded |
207.5 |
182.1 |
240.1 |
252.4 |
330.4 |
418.4 |
424.3 |
437.4 |
463.7 |
477.3 |
447.8 |
* Includes R01, R03,
U01, P01, and R37; R29 in 1994–2002; R55 in 1995–1996; R15 beginning
in 1996; R21 beginning in 1997; and R33 beginning in 2001.
† The number
for applications reviewed is based on preliminary data. |
NHLBI Competing Research Project
Grant Applications*: Fiscal Years 1995–2005
Number Reviewed and Awarded
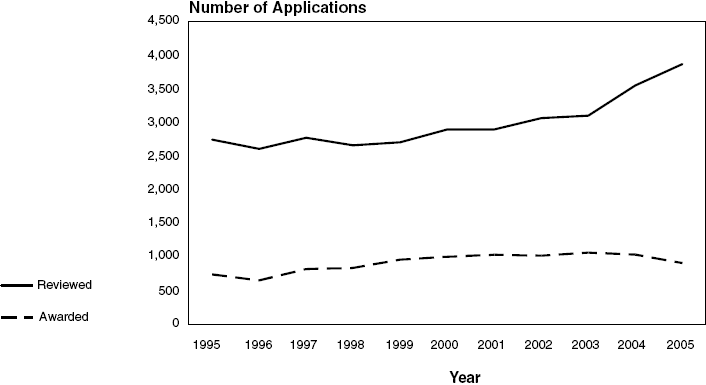
Text-only with data points are represented in the
table below.
| Number Reviewed
and Awarded and Percent Funded (Dollars in Millions) |
| |
Fiscal
Year |
| |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005† |
| Applications
Reviewed |
2,744 |
2,605 |
2,771 |
2,657 |
2,704 |
2,893 |
2,895 |
3,064 |
3,098 |
3,548 |
3,865 |
| RPGs Awarded |
740 |
652 |
821 |
837 |
959 |
1,003 |
1,033 |
1,018 |
1,064 |
1,034 |
909 |
| Success Rate
(percent) |
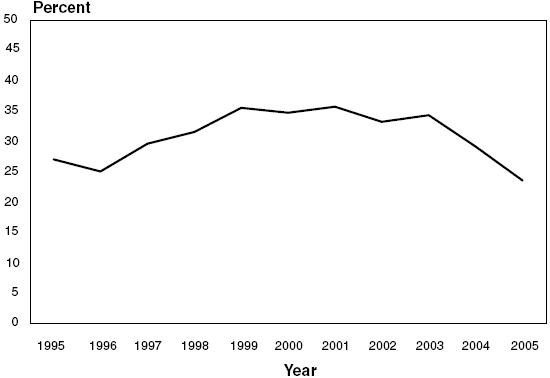
27.0 |
25.0 |
29.6 |
31.5 |
35.5 |
34.7 |
35.7 |
33.2 |
34.3 |
29.1 |
23.5 |
* Includes R01, R03,
U01, P01, and R37; R29 in 1994–2002; R55 in 1995–1996; R15 beginning
in 1996; R21 beginning in 1997; and R33 beginning in 2001.
† The number
of applications reviewed is based on preliminary data. |
Percent of Reviewed Applications
Funded (Success Rate)
Text-only with
data points
Back to Top
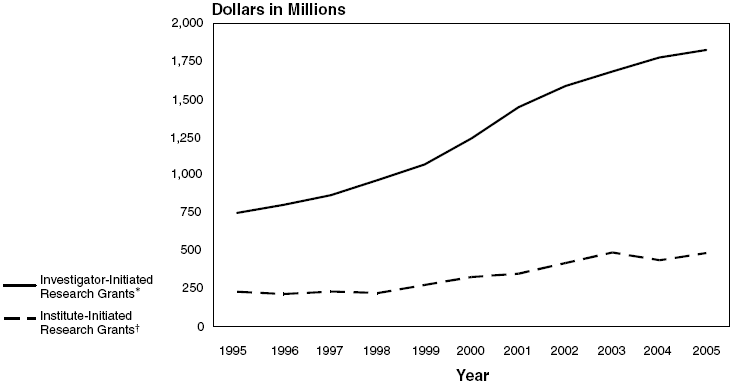
NHLBI Investigator-Initiated and
Institute-Initiated Grant Obligations: Fiscal Years 1995–2005
Text-only with data points are represented in the
table below.
NHLBI Investigator-Initiated and Institute-Initiated
Grant Obligations: Fiscal Years 1995–2005
| |
Dollars
(Millions) |
| |
Fiscal
Year |
| |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005 |
| Investigator-Initiated* |
$750.7 |
$804.1 |
$867.9 |
$966.6 |
$1,069.9 |
$1,241.6 |
$1,446.2 |
$1,584.9 |
$1,681.9 |
$1,773.4 |
$1,822.9 |
| Institute-Initiated† |
231.9 |
216.8 |
233.0 |
223.2 |
276.7 |
328.9 |
350.7 |
421.3 |
490.4 |
483.8 |
487.3 |
| Total |
$982.6 |
$1,020.9 |
$1,100.9 |
$1,189.8 |
$1,346.6 |
$1,570.5 |
$1,796.9 |
$2,006.2 |
$2,172.3 |
$2,257.2 |
$2,310.2 |
* Includes R01, R03,
U01, P01, R37, R41, R43, and R44; R29 in 1994–2002; R55 in 1995–1996;
R15 and R42 beginning in 1996; R21 beginning in 1997; and R33 beginning in
2001.
† Includes Centers Grants and Cooperative Agreement
RFAs.
‡ Excludes Program Evaluation Assessment of $4,435,000. |
Back to Top
NHLBI Research Project Grants*: Amount Funded by Type
of Award, Fiscal Years 1995–2005
| |
Dollars
(Millions) |
| |
Fiscal
Year |
| |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005 |
| Competing |
|
|
|
|
|
|
|
|
|
|
|
|
New Competing |
$111.1 |
$90.5 |
$135.8 |
$147.5 |
$202.0 |
$266.4 |
$280.0 |
$291.2 |
$285.5 |
$290.5 |
$270.0 |
|
Renewal Competing |
94.5 |
90.4 |
104.0 |
103.9 |
127.2 |
152.0 |
143.9 |
143.9 |
177.2 |
185.5 |
176.1 |
|
Competing
Supplements |
1.9 |
1.2 |
0.3 |
1.0 |
1.2 |
0.9 |
0.4 |
2.3 |
1.0 |
1.3 |
1.7 |
| Subtotal, Competing |
207.5 |
182.1 |
240.1 |
252.4 |
330.4 |
419.3 |
424.3 |
437.4 |
463.7 |
477.3 |
447.8 |
| Noncompeting |
|
|
|
|
|
|
|
|
|
|
|
| Subtotal,
Noncompeting |
588.4 |
649.9 |
662.4 |
721.3 |
770.6 |
889.3 |
1,101.5 |
1,281.3 |
1,390.3 |
1,454.9 |
1,520.0 |
| Total, Competing
and Noncompeting |
$795.9 |
$832.0 |
$902.5 |
$973.7 |
$1,101.0 |
$1,308.6 |
$1,525.8 |
$1,718.7 |
$1,854.0 |
$1,932.2 |
$1,967.8 |
| * Includes R01, R03,
U01, P01, and R37; R29 in 1994–2002; R55 in 1995–1996; R15 beginning
in 1996; R21 beginning in 1997; and R33 beginning in 2001. |
Facility and Administrative (F&A)* Costs of NHLBI
Research Project Grants†: Fiscal Years 1995–2005
| Dollars
(Thousands) |
| Fiscal Year |
Direct Cost |
F&A Cost † |
Total Cost |
F&A Cost as a Percent of Direct Cost |
| 1995 |
$543,502 |
$252,423 |
$795,925 |
46.4% |
| 1996 |
564,219 |
267,785 |
832,004 |
47.5 |
| 1997 |
611,576 |
290,915 |
902,491 |
47.6 |
| 1998 |
660,009 |
313,765 |
973,774 |
47.5 |
| 1999 |
764,198 |
336,756‡ |
1,100,954 |
44.1 |
| 2000 |
891,244 |
417,312 |
1,308,556 |
46.8 |
| 2001 |
1,045,144 |
480,673 |
1,525,817 |
46.0 |
| 2002 |
1,182,408 |
536,324 |
1,718,732 |
45.4 |
| 2003 |
1,276,819 |
577,131 |
1,853,950 |
45.2 |
| 2004 |
1,329,106 |
603,133 |
1,932,239 |
45.4 |
| 2005 |
1,355,803 |
612,007 |
1,967,810 |
45.1 |
* Previously called Indirect
Cost.
† Includes R01, R03, U01, P01, and R37; R29 in 1994–2002;
R55 in 1995–1996; R15 beginning in 1996; R21 beginning in 1997; and R33
beginning in 2001.
‡ Excludes Program Evaluation Assessment of
$1,216,000. |
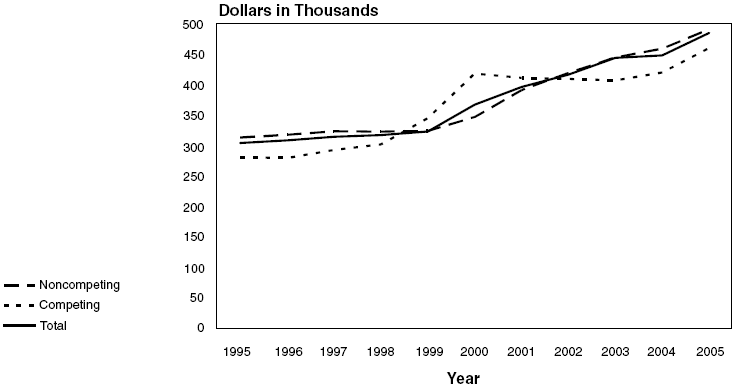
NHLBI Research Project Grants*:
Average Costs, Fiscal Years 1995–2005
Text-only with data points are represented in the
table below.
NHLBI Research Project Grants*: Average Costs, Fiscal
Years 1995–2005
| |
Dollars
(Thousands) |
| |
Fiscal
Year |
| |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005 |
| Noncompeting |
$312.8 |
$317.5 |
$323.0 |
$322.6 |
$323.4 |
$346.6 |
$390.7 |
$418.8 |
$444.4 |
$458.7 |
$490.6 |
| Competing |
280.4 |
279.3 |
292.5 |
301.6 |
344.5 |
418.0 |
410.8 |
409.1 |
406.7 |
419.7 |
459.9 |
| Total |
$303.7 |
$308.3 |
$314.2 |
$316.9 |
$329.4 |
$366.6 |
$396.1 |
$416.2 |
$433.8 |
$447.9 |
$484.8 |
| * Includes R01, R03,
U01, P01, R37, R41, R43, and R44; R29 in 1994–2002; R55 in 1995–1996;
R15 and R42 beginning in 1996; R21 beginning in 1997; and R33 beginning in
2001. |
Back to Top
NHLBI Cooperative Agreements (U01, U10) Programs
Cooperative Agreements were instituted to support
discrete, circumscribed projects in areas of an investigator’s specific
interest and competency with substantial programmatic participation by the
NHLBI during performance of the activity.
| |
Total Obligations Prior to FY 2005 |
Total FY 2005 Obligations |
Total Obligations to Date |
| Heart and
Vascular Diseases |
|
|
|
|
AIM HIGH: Niacin Plus Statin
To Prevent Vascular Events |
$ --- |
$663,376 |
$663,376 |
|
Atherosclerosis, Plaque, and
CVD in Communities |
4,099,685 |
5,145,549 |
9,245,234 |
|
Bypass Angioplasty
Revascularization Investigation in Type 2 Diabetics (BARI 2D) |
35,553,973 |
8,304,235 |
43,858,208 |
|
Cardiovascular Heart Study
(CHS) Events Follow-up Study |
--- |
1,007,645 |
1,007,645 |
|
Cardiovascular Outcomes in
Renal Atherosclerotic Lesions (CORAL) |
4,343,389 |
5,610,035 |
9,953,424 |
|
Center for Fetal Monkey Gene
Transfer for Heart, Lung, and Blood Diseases |
2,827,101 |
596,406 |
3,423,507 |
|
Claudication Exercise vs.
Edoluminal Revascularization |
--- |
1,368,413 |
1,368,413 |
|
Coronary Revascularization in
Diabetic Patients |
3,663,095 |
4,669,362 |
8,332,457 |
|
Dynamic Evaluation of
Percutaneous Coronary Intervention |
4,714,221 |
741,144 |
5,455,365 |
|
Family Blood Pressure
Program |
84,838,159 |
3,655,901 |
88,494,060 |
|
Genetics of Coronary Artery
Disease in Alaska Natives (GOCADAN) |
7,871,675 |
1,920,380 |
9,792,055 |
|
Girls Health Enrichment
Multisite Studies (GEMS) |
15,098,492 |
2,369,517 |
17,468,009 |
|
Heart Failure: A Controlled
Trial Investigating Outcomes of Exercise Training (HF-ACTION) |
25,044,553 |
4,483,140 |
29,527,693 |
|
Home Automatic External
Defibrillator Trial (HAT) |
13,263,462 |
1,800,742 |
15,064,384 |
|
IMMEDIATE Trial: Immediate
Myocardial Metabolic Enhancement During Initial Assessment and Treatment in
Emergency Care |
5,170,411 |
9,513,736 |
14,684,147 |
|
Interaction of Genes and
Environment in Shaping Risk Factors for Heart, Lung, Blood, and Sleep
Disorders |
35,525,298 |
10,547,590 |
46,072,888 |
|
Multidisciplinary Study of
Right Ventricular Dysplasia |
6,252,319 |
1,195,224 |
7,447,543 |
|
NHLBI Clinical Proteomics
Program |
--- |
5,103,660 |
5,103,660 |
|
Partnership Programs To Reduce
Cardiovascular Health Disparities |
6,468,544 |
7,202,208 |
13,670,752 |
|
Pediatric Cardiovascular
Clinical Research Network |
18,598,211 |
3,992,212 |
22,590,423 |
|
Pharmacogenetics Research
Network |
33,206,897 |
6,334,303 |
39,541,200 |
|
Preventing Overweight Using
Novel Dietary Strategies (POUNDS LOST) |
2,899,312 |
2,017,478 |
4,916,790 |
|
Primordial Prevention of
Overweight in American Indian Children |
--- |
354,427 |
354,427 |
|
Programs of Excellence in Gene
Therapy |
61,140,050 |
10,803,812 |
71,943,862 |
|
Programs of Excellence in
Nanotechnology |
--- |
6,322,873 |
6,322,873 |
|
Programs of Genomic
Applications (PGAs) for Heart, Lung, and Blood Diseases |
165,782,192 |
14,858,305 |
180,640,497 |
|
Resuscitation Outcome
Improvement Consortium |
6,886,109 |
9,338,606 |
16,224,715 |
|
Stop Atherosclerosis in Native
Diabetics Study (SANDS) |
6,681,337 |
2,323,642 |
9,004,979 |
|
Strong Heart Study |
46,438,304 |
5,300,000 |
51,738,304 |
|
Surgical Treatment for
Ischemic Heart Failure (STICH) |
13,864,647 |
6,082,190 |
13,864,647 |
|
Trial of Activity for
Adolescent Girls (TAAG) |
28,202,937 |
5,102,829 |
33,305,766 |
|
Weight Loss Maintenance
(WLM) |
8,054,488 |
3,098,894 |
11,153,382 |
|
Women’s Ischemia Syndrome
Evaluation (WISE) |
5,617,360 |
995,753 |
6,613,113 |
| Subtotal, Heart and
Vascular Diseases |
652,106,401 |
152,823,587 |
804,929,988 |
| Lung
Diseases |
|
|
|
|
Asthma Clinical Research
Network (ACRN), Phase II |
16,605,558 |
8,667,026 |
25,272,584 |
|
Centers for Reducing Asthma
Disparities |
17,269,191 |
5,135,604 |
22,404,795 |
|
Childhood Asthma Management
Program–Continuation Study (CAMP–CS)/Phase 2 |
3,532,802 |
2,622,721 |
6,155,523 |
|
Childhood Asthma Research and
Education (CARE) Network |
31,398,337 |
5,704,202 |
37,102,539 |
|
Collaborative Program in
Bronchopulmonary Dysplasia |
26,302,593 |
5,353,412 |
31,656,005 |
|
COPD Clinical Research
Network |
13,691,750 |
8,437,795 |
22,129,545 |
|
Early Antipseudomonal Therapy
in Cystic Fibrosis |
1,064,237 |
1,047,381 |
2,111,618 |
|
Idiopathic Pulmonary Fibrosis
Clinical Research Network |
--- |
3,486,226 |
3,486,226 |
|
Pharmacogenetics of Asthma
Treatment |
10,847,376 |
3,361,644 |
14,209,020 |
|
Prospective Investigation of
Pulmonary Embolism Diagnosis-III (PIOPED III) |
--- |
2,301,770 |
2,301,770 |
| Subtotal, Lung
Diseases |
120,711,844 |
46,117,781 |
166,829,625 |
| Blood Diseases
and Resources |
|
|
|
|
Blood and Marrow Transplant
Clinical Research Network |
23,181,808 |
6,459,743 |
29,641,551 |
|
Center for Human Cell
Therapy |
2,837,171 |
2,352,572 |
5,179,743 |
|
Functional Outcomes in
Cardiovascular Patients Undergoing Surgical Hip Fracture Repair
(FOCUS) |
3,435,202 |
2,922,730 |
6,357,932 |
|
Stroke With Transfusions
Changing to Hydroxyurea (SWITCH) |
--- |
3,345,345 |
3,345,345 |
|
Thalassemia (Cooley’s
Anemia) Clinical Research Network |
11,374,688 |
2,729,989 |
14,104,677 |
|
Transfusion
Medicine/Hemostasis Clinical Research Network |
18,386,476 |
6,221,140 |
24,607,616 |
| Subtotal, Blood Diseases
and Resources |
59,215,345 |
24,031,519 |
83,236,864 |
| National Center
on Sleep Disorders Research |
|
|
|
|
Apnea Positive Pressure
Long-Term Efficacy Study (APPLES) |
9,654,688 |
3,188,172 |
12,542,181 |
|
Sleep Heart Health
Study |
18,109,357 |
1,494,336 |
19,603,693 |
| Subtotal, National
Center on Sleep Disorders Research |
27,463,366 |
4,682,508 |
32,145,874 |
| Total, NHLBI
Cooperative Agreements |
$859,496,956 |
$227,655,395 |
$1,087,142,351 |
Back to Top
Heart and Vascular Diseases Program
AIM HIGH: Niacin Plus Statin To Prevent
Vascular Events, Initiated in Fiscal Year 2005
The purpose of this multicenter clinical trial is to
determine whether extended-release niacin plus simvastatin is superior to
simvastatin alone for preventing or delaying a major CVD event in patients with
atherogenic dyslipidemia. Niacin is used to raise HDL (“good”)
cholesterol and simvastatin is used to lower LDL (“bad”) cholesterol.
Twenty-seven percent of the population will be black.
Obligations
Funding History:
Fiscal Year
2005—$663,376
Total Funding to Date—$663,376
Current Active Organizations and Grant
Numbers
- University of Washington
Seattle,
Washington—HL-081616
- AXIO Research, LLC
Seattle,
Washington—HL-081649
Atherosclerosis, Plaque, and CVD in
Communities, Initiated in Fiscal Year 2004
The purpose of this study is to identify correlates of
atherosclerotic plaque characteristics and early changes in the vascular wall
in a subset of the biethnic Atherosclerosis Risk in Communities (ARIC) cohort.
Investigators will use stored DNA samples to test genomic correlates of plaque
characteristics and their ability to predict coronary heart disease and
stroke.
Obligations
Funding History:
Fiscal Year
2005—$5,145,549
Fiscal Year
2004—$4,099,685
Total Funding to Date—$9,245,234
| Current Active
Organization and Grant Number |
- University of Texas Health Science Center
Houston, Texas
|
— HL-075572 |
Back to Top
Bypass Angioplasty Revascularization Investigation in
Type 2 Diabetics (BARI 2D), Initiated in Fiscal Year 2000
The purpose of this trial is to compare alternative
treatment strategies for managing type 2 diabetic patients with
angiographically proven coronary artery disease and stable angina or ischemia.
Revascularization combined with aggressive medical anti-ischemia treatment will
be compared to aggressive medical anti-ischemia treatment alone;
simultaneously, researchers will determine whether insulin-sensitizing drugs
like metformin and the glitazones for controlling blood sugar level offer any
survival advantage over drugs that increase insulin level. Twenty percent of
the patients are from minority populations.
Obligations
Funding History:
Fiscal Year
2005—$8,304,235
Fiscal Years
2000–2004—$35,553,973
Total Funding to Date—$43,854,208
| Current Active
Organizations and Grant Numbers |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HL-061744 |
- St. Louis University
St. Louis,
Missouri
|
—HL-061746 |
- Stanford University
Stanford,
California
|
—HL-061748 |
- University of Vermont
Burlington,
Vermont
|
—HL-063804 |
Cardiovascular Heart Study (CHS) Events Follow-Up
Study, Initiated in Fiscal Year 2005
The purpose of this project is to enhance the use of
data and samples from the CHS during a transition period from the current
contract-funded, NHLBI-directed program to one directed by a steering committee
of investigators with independently acquired grants. During the follow-up, many
of the initial exam components will be repeated and CVD events will be
recorded. The data and specimens collected in CHS represent a major national
resource for the study of health, aging, and CVD in older adults. Seventeen
percent of the participants are from minority populations.
Obligations
Funding History:
Fiscal Year
2005—$1,007,645
Total Funding to Date—$1,007,645
| Current Active
Organization and Grant Number |
- University of Washington
Seattle,
Washington
|
—HL-080295 |
Back to Top
Cardiovascular Outcomes in Renal Atherosclerotic
Lesions (CORAL), Initiated in Fiscal Year 2004
The purpose of this trial is to determine whether
revascularization of a stenotic renal artery plus medical therapy is associated
with improved clinical outcomes compared with medical therapy alone. Thirty
percent of the participants will be black.
Obligations
Funding History:Fiscal Year
2005—$5,610,035
Fiscal Year
2004—$4,343,389
Total Funding to Date—$9,953,424
| Current Active
Organizations and Grant Numbers |
- Medical College of Ohio
Toledo, Ohio
|
—HL-071556 |
- University of Minnesota, Twin
Cities
Minneapolis, Minnesota
|
—HL-072734 |
- University of Virginia
Charlottesville,
Virginia
|
—HL-072735 |
- Beth Israel Deaconess Medical
Center
Boston, Massachusetts
|
—HL-072736 |
- Brigham and Women’s Hospital
Boston,
Massachusetts
|
—HL-072737 |
Center for Fetal Monkey Gene Transfer for Heart,
Lung, and Blood Diseases, Initiated in Fiscal Year 2001
The purpose of this Center is to provide expertise,
sources, and resources to NHLBI-supported investigators who wish to evaluate
viral and nonviral gene transfer strategies in nonhuman primates.
Obligations
Funding History:
Fiscal Year
2005—$596,406
Fiscal Years
2001–2004—$2,827,101
Total Funding to Date—$3,423,507
| Current Active
Organization and Grant Number |
- University of California, Davis
Davis,
California
|
—HL-069748 |
Claudication Exercise vs. Edoluminal
Revascularization, Initiated in Fiscal Year 2005
The purpose of this study is to test the hypothesis
that a strategy of aortoiliac stenting and pharmacotherapy improves maximum
walking duration better than a strategy of supervised rehabilitation, exercise,
and pharmacotherapy for those with aortoiliac artery obstruction at 6 months.
Other objectives are to compare the two treatment groups with a third group,
usual care and pharmacotherapy, at 6 months, and to compare maximum walking
duration change scores at 18 months, changes in free living daily activity
levels, and patient-perceived quality of life among all three groups.
Obligations
Funding History:
Fiscal Year
2005—$1,368,413
Total Funding to Date—$1,368,413
| Current Active
Organizations and Grant Numbers |
- Rhode Island Hospital
Providence, Rhode
Island
|
—HL-077221 |
- Brigham and Women’s Hospital
Boston,
Massachusetts
|
—HL-081658 |
Coronary Revascularization in Diabetic Patients,
Initiated in Fiscal Year 2004
The purpose of this clinical trial is to compare a
multivessel stenting strategy using the Sirolimus-eluting stents with CABG in
diabetic patients with multivessel disease. The primary outcome is the
difference in mortality rates between the stent group and the CABG group during
a 5-year period.
Obligations
Funding History:
Fiscal Year
2005—$4,669,362
Fiscal Year
2004—$3,663,095
Total Funding to Date—$8,332,457
| Current Active
Organization and Grant Number |
- Mount Sinai School of Medicine
New York,
New York
|
—HL-071988 |
Back to Top
Dynamic Evaluation of Percutaneous Coronary
Intervention, Initiated in Fiscal Year 1997
This program, which complements prior NHLBI
percutaneous transluminal coronary angioplasty (PTCA) registries and the New
Approaches to Coronary Intervention Registry, is evaluating patterns of device
usage, as well as immediate and follow-up outcomes in patients undergoing
percutaneous transluminal coronary revascularization. Results will provide
guidance to the cardiology community in selecting appropriate therapies and in
designing clinical trials to evaluate competing devices.
Obligations
Funding History:
Fiscal Year
2005—$741,144
Fiscal Years
1997–2004—$4,714,221
Total Funding to Date—$5,455,365
| Current Active
Organization and Grant Number |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HL-033292 |
Family Blood Pressure Program, Initiated in Fiscal
Year 1995
The objectives of this program are to identify major
genes associated with high blood pressure and to investigate the interactions
between genetic and environmental determinants of hypertension in defined
populations, many of which consist of specific minority groups. The study
consists of collaborative networks that share technology, data, skills,
biological materials, and population resources.
Obligations
Funding History:
Fiscal Year
2005—$3,655,901
Fiscal Years
1995–2004—$84,838,159
Total Funding to Date—$88,494,060
| Current Active
Organizations and Grant Numbers |
- University of Utah
Salt Lake City,
Utah
|
—HL-054471 |
- Washington University
St. Louis,
Missouri
|
—HL-054473 |
- University of Texas Health Science
Center
Houston, Texas
|
—HL-054481 |
- Staub Pacific Health Foundation Health
Research Institute
Honolulu, Hawaii
|
—HL-054498 |
- University of Michigan at Ann Arbor
Ann
Arbor, Michigan
|
—HL-054512 |
Genetics of Coronary Artery Disease in Alaska Natives
(GOCADAN), Initiated in Fiscal Year 2000
The purpose of this study is to document CVD and CVD
risk factors in approximately 40 extended families (1,214 members from villages
in Northern Alaska). Scientists seek to identify and characterize genes that
contribute to CVD in this unique and understudied population.
Obligations
Funding History:
Fiscal Year
2005—$1,920,380
Fiscal Years
2000–2004—$7,871,675
Total Funding to Date—$9,792,055
| Current Active
Organizations and Grant Numbers |
- MedStar Research Institute
Washington,
DC
|
—HL-064244 |
- Norton Sound Health Corporation
Nome,
Alaska
|
—HL-082458 |
- Southwest Foundation for Biomedical Research
San Antonio, Texas
|
—HL-082490 |
Girls Health Enrichment Multisite Studies (GEMS),
Initiated in Fiscal Year 1999
The objective of this project is to develop and test
interventions to prevent obesity by decreasing weight gain during the high-risk
transitional period from pre-puberty to puberty in black girls who are at risk
for developing obesity. Phase 1 (developmental and pilot studies) was completed
in FY 2002. Two sites began Phase 2 trials in FY 2003.
Obligations
Funding History:
Fiscal Year
2005—$2,369,517
Fiscal Years
1999–2004—$15,098,492
Total Funding to Date—$17,468,009
| Current Active
Organizations and Grant Numbers |
- University of Memphis
Memphis,
Tennessee
|
—HL-062662 |
- Stanford University
Stanford,
Califorinia
|
—HL-062663 |
Heart Failure: A Controlled Trial Investigating
Outcomes of Exercise (HF-ACTION), Initiated in Fiscal Year 2002
The purpose of this trial is to determine the
long-term safety and effectiveness of exercise training for patients with heart
failure. Patients receiving the exercise regimen also will receive standard
care and will be compared with patients receiving standard care alone.
Obligations
Funding History:
Fiscal Year
2005—$4,483,140
Fiscal Years
2002–2004—$25,044,553
Total Funding to Date—$29,527,693
| Current Active
Organizations and Grant Numbers |
- Duke University
Durham, North
Carolina
|
—HL-063747 |
- Case Western Reserve University Henry Ford
Health System
Detroit, Michigan
|
—HL-064250 |
- Oregon Health & Science
University
Portland, Oregon
|
—HL-064257 |
- Washington University
St. Louis,
Missouri
|
—HL-064264 |
- University of Colorado Health Sciences
Center
Denver, Colorado
|
—HL-064265 |
- Duke University
Durham, North
Carolina
|
—HL-066461 |
- Emory University
Atlanta, Georgia
|
—HL-066482 |
- Wake Forest University
Winston-Salem,
North Carolina
|
—HL-066491 |
- Ohio State University
Columbus, Ohio
|
—HL-066494 |
- University of Alabama at
Birmingham
Birmingham, Alabama
|
—HL-066497 |
- Case Western Reserve University
Cleveland,
Ohio
|
—HL-066501 |
- Boston Medical Center
Boston,
Massachusetts
|
—HL-068973 |
- University of California, Los Angeles
Los
Angeles, California
|
—HL-068980 |
Home Automatic External Defibrillator Trial (HAT),
Initiated in Fiscal Year 2002
The purpose of this trial is to compare standard
response (call 9–1–1 and give cardiopulmonary resuscitation) to
sudden cardiac arrest to standard response augmented with automatic external
defibrillator use provided by a spouse or other family member in 7,000
survivors of an anterior wall MI. The primary end point is mortality.
Obligations
Funding History:
Fiscal Year
2005—$1,800,742
Fiscal Years
2002–2004—$13,263,642
Total Funding to Date—$15,064,384
| Current Active
Organization and Grant Number |
- Seattle Institute for Cardiac
Research
Seattle, Washington
|
—HL-067972 |
IMMEDIATE Trial: Immediate Myocardial Metabolic
Enhancement During Initial Assessment and Treatment in Emergency Care,
Initiated in Fiscal Year 2004
The purpose of this program is to study the effects of
early administration of glucose, insulin, and potassium (GIK) in reducing
mortality in patients from acute coronary syndrome (ACS). Patients experiencing
an ACS (including acute MI and unstable angina pectoris) will be treated with
GIK as soon as possible in prehospital emergency medical service settings or
immediately upon arrival for those presenting to emergency departments.
Obligations
Funding History:
Fiscal Year
2005—$9,513,736
Fiscal Year
2004—$5,170,411
Total Funding to Date—$14,684,147
| Current Active
Organizations and Grant Numbers |
- New England Medical Center
Hospitals
Boston, Massachusetts
|
—HL-077821 |
- New England Medical Center
Hospitals
Boston, Massachusetts
|
—HL-077822 |
- New England Medical Center
Hospitals
Boston, Massachusetts
|
—HL-077823 |
- New England Medical Center
Hospitals
Boston, Massachusetts
|
—HL-077826 |
Back to Top
Interaction of Genes and Environment in Shaping Risk
Factors for Heart, Lung, and Blood Diseases and Sleep Disorders, Initiated in
Fiscal Year 2002
The purpose of this study is to identify novel genes
that interact with specific environmental exposures to modify risk factors for
heart, lung, and blood diseases and sleep disorders. The genetic aspects of
response to environmental change and related biological mechanisms will be
studied using short-term, focused interventions in families. Subgroups will be
identified based on genotypes that are most likely to benefit from targeted
environmental changes designed to reduce the development or progression of
heart, lung, and blood diseases or sleep disorders.
Obligations
Funding History:
Fiscal Year
2005—$10,547,590
Fiscal Years
2002–2004—$35,525,298
Total Funding to Date—$46,072,888
| Current Active
Organizations and Grant Numbers |
- Tulane University
New Orleans,
Louisiana
|
—HL-072507 |
- LSU Pennington Biomedical Research
Center
Baton Rouge, Louisiana
|
—HL-072510 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-072518 |
- University of Minnesota, Twin
Cities
Minneapolis, Minnesota
|
—HL-072524 |
- University of Maryland Baltimore Professional
School
Baltimore, Maryland
|
—HL-072525 |
Multidisciplinary Study of Right Ventricular
Dysplasia, Initiated in Fiscal Year 2001
The purpose of this multidisciplinary, multicenter
study is to investigate the cardiac, clinical, and genetic aspects of
arrhythmogenic right ventricular dysplasia (ARVD). A North American ARVD
registry of patients and their families will be established. Researchers seek
to identify chromosomal loci and specific genetic mutations associated with
this disorder.
Obligations
Funding History:
Fiscal Year
2005—$1,195,224
Fiscal Years
2001–2004—$6,252,319
Total Funding to Date—$7,447,543
| Current Active
Organizations and Grant Numbers |
- University of Arizona
Tucson, Arizona
|
—HL-065594 |
- Baylor College of Medicine
Houston,
Texas
|
—HL-065652 |
- University of Rochester
Rochester, New
York
|
—HL-065691 |
NHLBI Clinical Proteomics Program, Initiated in
Fiscal Year 2005
The purpose of this program is to promote systematic,
comprehensive, large-scale validation of existing and new candidate protein
markers that are appropriate for routine use in the diagnosis and management of
heart, lung, and blood diseases and sleep disorders. The Program will
facilitate validation of protein panels that may be used to predict disease
susceptibility or to assist in differential diagnosis, disease staging,
selection of individualized therapies, or monitoring of treatment responses. It
will also establish a high-quality education and skills development program to
ensure that scientists develop the expertise needed to address the complex,
multifaceted challenges in clinical proteomics.
Obligations
Funding History:
Fiscal Year
2005—$5,103,660
Total Funding to Date—$5,103,660
| Current Active
Organizations and Grant Numbers |
- Mayo Clinic
Rochester, Minnisota
|
—HL-081331 |
- Vanderbilt University
Nashville,
Tennessee
|
—HL-081332 |
- University of Colorado, Denver
Denver,
Colorado
|
—HL-081335 |
- Masschusetts General Hospital
Boston,
Massachusetts
|
—HL-081341 |
Partnership Programs To Reduce Cardiovascular Health
Disparities, Initiated in Fiscal Year 2004
The objectives of this study are to improve the
provider and patient approaches to treatment of hypertension and diabetes,
modify physician-related barriers to minority enrollment in clinical trials,
improve patient adherence to treatment plans, and build sustainable research
programs at minority-serving institutions.
Obligations
Funding History:
Fiscal Year
2005—$7,202,208
Fiscal Year
2004—$6,468,544
Total Funding to Date—$13,670,752
| Current Active
Organizations and Grant Numbers |
- Bon Secours Hospital Baltimore,
Inc.
Baltimore, Maryland
|
—HL-079150 |
- University of Maryland Baltimore Professional
School
Baltimore, Maryland
|
—HL-079151 |
- Queen’s Medical Center
Honolulu,
Hawaii
|
—HL-079152 |
- Cooper Green Hospital
(Birmingham)
Birmingham, Alabama
|
—HL-079153 |
- Emory University
Atlanta, Georgia
|
—HL-079156 |
- Denver Health and Hospital
Authority
Denver, Colorado
|
—HL-079160 |
- University of Hawaii at Manoa
Honolulu,
Hawaii
|
—HL-079163 |
- University of Alabama at
Birmingham
Birmingham, Alabama
|
—HL-079171 |
- University of Colorado Health Sciences
Center
Denver, Colorado
|
—HL-079208 |
- Morehouse School of Medicine
Atlanta,
Georgia
|
—HL-079214 |
- Jackson Hinds Comprehensive Health
Center
Jackson, Mississippi
|
—HL-079378 |
- University of Mississippi Medical Center
Jackson, Mississippi
|
—HL-079458 |
Back to Top
Pediatric Cardiovascular Clinical Research Network,
Initiated in Fiscal Year 2001
See Chapter 11. Clinical
Trials.
Pharmacogenetics Research Network, Initiated in
Fiscal Year 2001
The purpose of this study is to establish a network to
systematically evaluate candidate genes that may influence pharmacologic
response to drug treatments for arrhythmia, heart failure, hypertension, and
lipid disorders. Investigators seek to identify gene polymorphisms capable of
predicting drug toxicity and efficacy. One of the projects has 50 percent
minority participation.
Obligations
Funding History:
Fiscal Year
2005—$6,334,303
Fiscal Years
2001–2004—$33,206,897
Total Funding to Date—$39,541,200
| Current Active
Organizations and Grant Numbers |
- Vanderbilt University
Nashville,
Tennessee
|
—HL-065962 |
- Children’s Hospital and Research Center
at Oakland
Oakland, California
|
—HL-069757 |
- Stanford University
Stanford,
California
|
—GM-061374 |
Preventing Overweight Using Novel Dietary Strategies
(POUNDS LOST), Initiated in Fiscal Year 2003
The purpose of this study is to compare the effects of
four diets low in saturated fat and differing in macronutrient composition on
weight loss and its maintenance in 800 overweight or obese adults. The diet
consists of moderate fat (40 percent energy) or low fat (20 percent energy)
with two different protein levels (15 and 25 percent). Approximately 20 percent
of the participants will be minority.
Obligations
Funding History:
Fiscal Year
2005—$2,017,478
Fiscal Years
2003–2004—$2,899,312
Total Funding to Date—$4,916,790
| Current Active
Organization and Grant Number |
- Harvard School of Public Health
Boston,
Massachusetts
|
—HL-073286 |
Primordial Prevention of Overweight in American
Indian Children, Initiated in Fiscal Year 2005
The purpose of this study is to prevent early
childhood overweight in American Indian children. A cohort of children born
over an 18-month period will be randomized to either a control or intervention
condition. The intervention comprises a community-wide intervention coupled
with individualized family counseling to improve nutrition and physical
activity in infants and toddlers. A central feature of the project is to
develop and test culturally appropriate interventions that can be incorporated
into clinical programs of the community health care systems or delivered
through public health approaches in Native communities.
Obligations
Funding History:
Fiscal Year
2005—$354,427
Total Funding to Date—$354,427
| Current Active
Organization and Grant Number |
- Kaiser Foundation Research Institutes
Oakland, California
|
—HL-081624 |
Back to Top
Programs of Excellence in Gene Therapy, Initiated in
Fiscal Year 2000
The objective of these programs is to create an
environment that will enable rapid translation of preclinical studies in
cardiovascular, pulmonary, and hematologic diseases into human pilot
experiments. In addition, the programs are offering training at the interface
between basic science and clinical application. Six national cores provide
access to specialized services, such as generating vectors for clinical use,
performing morphologically based studies, producing and processing
hematopoietic stem cells, and performing primate transplantation studies.
Obligations
Funding History:
Fiscal Year
2005—$10,803,812
Fiscal Years
2000–2004—$61,140,050
Total Funding to Date—$71,943,862
| Current Active
Organizations and Grant Numbers |
- University of Washington
Seattle,
Washington
|
—HL-066947 |
- Stanford University
Stanford,
California
|
—HL-066948 |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HL-066949 |
- Weill Medical College of Cornell
University
New York, New York
|
—HL-066952 |
- Weill Medical College of Cornell
University
New York, New York
|
—HL-067738 |
Programs of Excellence in Nanotechnology, Initiated
in Fiscal Year 2005
The purpose of this program is to establish
multidisciplinary teams to develp nanotechnology and biomolecular engineering
tools and methodologies to detect and analyze atherosclerotic plaque formation.
The program presents an unique opportunity for research collaboration and
skills training by bring bioengineering and nanotechnology solutions into
medicine and vice versa.
Obligations
Funding History:
Fiscal Year
2005—$6,322,873
Total Funding to Date—$6,322,873
| Current Active
Organization and Grant Number |
- Emory University
Atlanta, Georgia
|
—HL-080711 |
Programs of Genomic Applications (PGAs) for Heart,
Lung, and Blood Diseases, Initiated in Fiscal Year 2000
The goal of this program is to develop information,
tools, and resources to link genes to biological function. Specifically,
researchers seek to identify human genes relevant to heart, lung, blood, and
sleep functions. In addition, the PGAs will establish training programs for
NHLBI-supported investigators in the use of genomic information and
technologies.
Obligations
Funding History:
Fiscal Year
2005—$14,858,305
Fiscal Years
2000–2004—$165,782,192
Total Funding to Date—$180,640,497
| Current Active
Organizations and Grant Numbers |
- Medical College of Wisconsin
Milwaukee,
Wisconsin
|
—HL-066579 |
- University of California, San
Francisco
San Francisco, California
|
—HL-066600 |
- Jackson Laboratory
Bar Harbor, Maine
|
—HL-066611 |
- University of California
Los Angeles,
California
|
—HL-066621 |
- University of Washington
Seattle,
Washington
|
—HL-066642 |
- University of California Lawrence Berkeley
Laboratory
Berkeley, California
|
—HL-066681 |
- University of Washington
Seattle,
Washington
|
—HL-066682 |
Resuscitation Outcome Improvement Consortium,
Initiated in Fiscal Year 2004
The purpose of this program is to establish a
resuscitation research consortium to conduct clinical research in the areas of
cardiopulmonary arrest and traumatic injury leading to arrest. The consortium
will enable investigators to conduct multiple collaborative trials to expedite
the translation of promising scientific and clinical advances to improve
resuscitation outcomes.
Obligations
Funding History:
Fiscal Year
2005—$9,338,606
Fiscal Year
2004—$6,886,109
Total Funding to Date—$16,224,715
| Current Active
Organizations and Grant Numbers |
- University of Washington
Seattle,
Washington
|
—HL-077863 |
- University of Iowa
Iowa City, Iowa
|
—HL-077865 |
- Medical College of Wisconsin
Milwaukee,
Wisconsin
|
—HL-077866 |
- University of Washington
Seattle,
Washington
|
—HL-077867 |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HL-077871 |
- St. Michael’s Hospital
Toronto,
Ontario
|
—HL-077872 |
- Oregon Health & Science
University
Portland, Oregon
|
—HL-077873 |
- University of Alabama at
Birmingham
Birmingham, Alabama
|
—HL-077881 |
- Ottawa Health Research Institute
Ottawa,
Ontario
|
—HL-077885 |
- University of Texas Southwestern Medical
Center
Dallas, Texas
|
—HL-077887 |
- University of California, San Diego
La
Jolla, California
|
—HL-077908 |
Back to Top
Stop Atherosclerosis in Native Diabetics Study
(SANDS), Initiated in Fiscal Year 2002
This study will address the high incidence of
cardiovascular disease in a population with a high prevalence of diabetes, but
relatively low levels of LDL cholesterol and blood pressure. It will compare
aggressive lowering of LDL cholesterol and blood pressure to the usual care
standard.
Obligations
Funding History:
Fiscal Year
2005—$2,323,642
Fiscal Years
2002–2004—$6,681,337
Total Funding to Date—$9,004,979
| Current Active
Organization and Grant Number |
- MedStar Research Institute
Washington,
DC
|
—HL-067031 |
Strong Heart Study, Initiated in Fiscal Year
1988
The objectives of this study are to survey CVD
morbidity and mortality rates among three geographically diverse groups of
American Indians and to estimate their levels of CVD risk factors. Phases II
and III of the cohort study extended surveillance of community mortality and
assessed development of CVD and changes in CVD risk factors. In Phase III,
investigators added a substudy of asthma and a pilot family study. The purpose
of Phase IV is to enlarge the family study to 120 families comprising 3,600
members to investigate genetic and environmental contributors of CVD.
Obligations
Funding History:
Fiscal Year
2005—$5,300,000
Fiscal Years
1988–2004—$46,438,304
Total Funding to Date—$51,738,304
| Current Active
Organizations and Grant Numbers |
- MedStar Research Institute
Washington,
DC
|
—HL-041642 |
- Missouri Breaks Research, Inc.
Timberlake,
South Dakota
|
—HL-041652 |
- University of Oklahoma Health Sciences
Center
Oklahoma City, Oklahoma
|
—HL-041654 |
- Southwest Foundation for Biomedical
Research
San Antonio, Texas
|
—HL-065520 |
- Weill Medical College of Cornell
University
New York, New York
|
—HL-065521 |
Surgical Treatment for Ischemic Heart Failure
(STICH), Initiated in Fiscal Year 2002
The purpose of this clinical trial is to determine
whether CABG plus intensive medical therapy improves long-term survival of
patients with heart failure and left ventricular (LV) dysfunction who have
coronary artery disease amenable to surgical revascularization, compared to
medical therapy alone; and to determine whether CABG plus surgical ventricular
restoration to a more normal LV size improves survival free of subsequent
hospitalizations of patients with anterior LV dysfunction, compared to CABG
alone.
Obligations:
Funding History:
Fiscal Year
2005—$6,082,190
Fiscal Years
2002–2004—$13,864,647
Total Funding to Date—$19,946,837
| Current Active
Organizations and Grant Numbers |
- Thomas Jefferson University
Philadelphia,
Pennsylvania
|
—HL-069009 |
- Mayo Clinic
Rochester, Minnesota
|
—HL-069010 |
- Duke University
Durham, North
Carolina
|
—HL-069011 |
- Northwestern University
Chicago,
Illinois
|
—HL-069012 |
- Duke University
Durham, North
Carolina
|
—HL-069013 |
- Duke University
Durham, North
Carolina
|
—HL-069015 |
- University of Southern California
Los
Angeles, California
|
—HL-072683 |
Trial of Activity for Adolescent Girls (TAAG),
Initiated in Fiscal Year 2000
See Chapter 11. Clinical
Trials.
Weight Loss Maintenance (WLM), Initiated in Fiscal
Year 2003
The purpose of this multicenter trial is to evaluate
the effectiveness of two strategies to maintain weight loss for 2½ years
in approximately 800 overweight or obese adults. Individuals who are taking
medication for hypertension of dyslipidemia or who are diabetic enter a 6-month
weight program. Those who lose at least 9 pounds are randomized into one of
three groups: one that provides monthly personal contacts with a trained
interventionist, primarily by telephone; one that provides frequent contacts
through an interactive Web-based program; or usual care. Forty percent of the
participants will be black.
Obligations
Funding History:
Fiscal Year
2005—$3,098,894
Fiscal Years
2003–2004—$8,054,488
Total Funding to Date—$11,153,382
| Current Active
Organizations and Grant Numbers |
- Center for Health Research
Portland,
Oregon
|
—HL-068676 |
- Duke Hypertensive Center
Durham, North
Carolina
|
—HL-068734 |
- Center for Health Research
Portland,
Oregon
|
—HL-068790 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-068920 |
- LSU Pennington Biomedical Research
Center
Baton Rouge, Louisiana
|
—HL-068955 |
Women’s Ischemia Syndrome Evaluation (WISE),
Initiated in Fiscal Year 2001
The purpose of this study is to extend the follow-up
of WISE patients to determine the incremental long-term prognostic value of
novel testing developed in WISE, develop sex-specific incremental outcome
models to evaluate the prognostic value of female reproductive variables, and
maintain a WISE database and infrastructure to facilitate further
investigations into the mechanisms underlying ischemic syndromes in women.
Obligations
Funding History:
Fiscal Year
2005—$995,753
Fiscal Years
2001–2004—$5,617,360
Total Funding to Date—$6,613,113
| Current Active
Organization and Grant Number |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HL-064829 |
- University of Florida
Gainesville,
Florida
|
—HL-064924 |
Back to Top
Lung Diseases Program
Asthma Clinical Research Network (ACRN) Phase II,
Initiated in Fiscal Year 2003
See Chapter 11. Clinical
Trials.
Centers for Reducing Asthma Disparities, Initiated in
Fiscal Year 2002
The purpose of this study is to establish cooperative
centers of research to reduce asthma disparities between whites and minorities
and economically disadvantaged populations. The mission of the centers,
comprising partnerships between minority-servicing medical institutions and
research-intensive institutions, is to promote interdisciplinary investigation
of factors that contribute to disparities in asthma, accelerate development and
evaluation of strategies to promote effective asthma management among minority
and economically disadvantaged populations, encourage training and career
development for minority clinical research investigators, and improve the
effectiveness of NHLBI-supported research-intensive institutions in developing
and sustaining culturally appropriate research and demonstration activities on
reducing disparities.
Obligations
Funding History:
Fiscal Year
2005—$5,135,604
Fiscal Years
2002–2004—$17,269,191
Total Funding to Date—$22,404,795
| Current Active
Organizations and Grant Numbers |
- Meharry Medical College
Nashville,
Tennessee
|
—HL-072431 |
- Howard University
Washington, DC
|
—HL-072433 |
- Rhode Island Hospital
Providence, Rhode
Island
|
—HL-072438 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-072455 |
- Vanderbilt University
Nashville,
Tennessee
|
—HL-072471 |
- Northwestern University
Chicago,
Illinois
|
—HL-072478 |
- Hektoen Institute for Medical Research
Chicago, Illinois
|
—HL-072496 |
- University of Puerto Rico Medical
Sciences
San Juan, Puerto Rico
|
—HL-072519 |
Childhood Asthma Management Program–Continuation
Study (CAMP–CS)/Phase 2, Initiated in Fiscal Year 2003
The objectives of this observational study are to
follow the original CAMP cohort for 4 more years into early adulthood to
determine the effects of long-term (3.5 to 5.5 years) corticosteroid therapy,
started at ages 5 to 12, on outcomes of pulmonary function, height, bone
density, and clinical course of asthma; 31 percent of the participants are from
minority groups.
Obligations
Funding History:
Fiscal Year
2005—$2,622,721
Fiscal Years
2003–2004—$3,532,802
Total Funding to Date—$6,155,523
| Current Active
Organizations and Grant Numbers |
- Washington University
St. Louis,
Missouri
|
—HL-075232 |
- Hospital for Sick Children
Toronto,
Ontario
|
—HL-075407 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-075408 |
- Asthma, Inc.
Seattle, Washington
|
—HL-075409 |
- University of California, San Diego
La
Jolla, California
|
—HL-075415 |
- National Jewish Medical and Research
Center
Denver, Colorado
|
—HL-075416 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-075417 |
- University of Puerto Rico Medical
Sciences
San Juan, Puerto Rico
|
—HL-075419 |
- University of New Mexico
Albuquerque, New
Mexico
|
—HL-075420 |
Childhood Asthma Research and Education (CARE)
Network, Initiated in Fiscal Year 1999
See Chapter 11. Clinical
Trials.
Back to Top
Collaborative Program in Bronchopulmonary Dysplasia,
Initiated in Fiscal Year 1999
The objectives of this program are to support a
multi-institutional collaborative research effort by providing a well-defined
model of prematurity and bronchopulmonary dysplasia to investigators, and to
study mechanisms of lung pathobiology that underlie development of chronic lung
disease of prematurity.
Obligations
Funding History:
Fiscal Year
2005—$5,353,412
Fiscal Years
1999–2004—$26,302,593
Total Funding to Date—$31,656,005
| Current Active
Organizations and Grant Numbers |
- Southwest Foundation for Biomedical Research
San Antonio, Texas
|
—HL-052636 |
- Brigham and Women’s Hospital
Boston,
Massachusetts
|
—HL-052638 |
- University of California, San
Francisco
San Francisco, California
|
—HL-056061 |
- National Jewish Medical and Research
Center
Denver, Colorado
|
—HL-056263 |
- Barnes Jewish Hospital
St. Louis,
Missouri
|
—HL-063387 |
- National Jewish Medical and Research
Center
Denver, Colorado
|
—HL-063397 |
- University of Texas Southwestern Medical
Center
Dallas, Texas
|
—HL-063399 |
- University of Rochester
Rochester, New
York
|
—HL-063400 |
- Children’s Hospital of
Philadelphia
Philadelphia, Pennsylvania
|
—HL-075900 |
- Children’s Hospital
Boston,
Massachusetts
|
—HL-075904 |
COPD Clinical Research Network, Initiated in Fiscal
Year 2003
See Chapter 11. Clinical
Trials.
Early Antipseudomonal Therapy in Cystic Fibrosis,
Initiated in Fiscal Year 2004
The purpose of this study is to determine a safe,
effective, and systematic approach for treating young children (ages 1 to 12
years) with cystic fibrosis who are found to be infected with Pseudomonas
aemginosa (Pa). The goal is to intervene with antipseudomonal therapy at the
first isolation of Pa to delay or prevent chronic infections that lead to
irreversible lung destruction.
Obligations
Funding History:
Fiscal Year
2005—$1,047,381
Fiscal Year
2004—$1,064,237
Total Funding to Date—$2,111,618
| Current Active
Organization and Grant Number |
- Children’s Hospital and Regional Medical
Center
Seattle, Washington
|
—HL-080310 |
Idiopathic Pulmonary Fibrosis Clinical Research
Network, Initiated in Fiscal Year 2005
See Chapter 11. Clinical
Trials.
Pharmacogenetics of Asthma Treatment, Initiated in
Fiscal Year 2000
The objective of this project is to bring together
research experts in asthma, epidemiology, statistics, bioinformatics,
physiology, clinical trials, genetics, and genomics to focus on the
pharmacogenetics of asthma treatment.
Obligations
Funding History:
Fiscal Year
2005—$3,361,644
Fiscal Years
2000–2004—$10,847,376
Total Funding to Date—$14,209,020
| Current Active
Organization and Grant Number |
- Brigham and Women’s Hospital
Boston,
Massachusetts
|
—HL-065899 |
Prospective Investigation of Pulmonary Embolism
Diagnosis-III (PIOPED III), Initiated in Fiscal Year 2005
The purpose of this study is to determine the
diagnostic accuracy of gadolinium-enhanced magnetic resonance angiography of
the pulmonary arteries in combination with magnetic resonance venography of the
lower extremities for the detection of acute venous thromboembolic disease.
Obligations
Funding History:
Fiscal Year
2005—$2,301,770
Total Funding to Date—$2,301,770
| Current Active
Organizations and Grant Numbers |
- Massachusetts General Hospital
Boston,
Massachusetts
|
—HL-077149 |
- University of Michigan
Ann Arbor,
Michigan
|
—HL-077150 |
- University of Calgary
Calgary,
Alberta
|
—HL-077151 |
- Emory University
Atlanta, Georgia
|
—HL-077153 |
- Washington University
St. Louis,
Missouri
|
—HL-077154 |
- George Washington University
Washington,
D.C.
|
—HL-077155 |
- St. Joseph Mercy-Oakland
Pontiac,
Michigan
|
—HL-077358 |
- New York University
New York, New
York
|
—HL-081593 |
- St. Joseph Mercy-Oakland
Pontiac,
Michigan
|
—HL-081594 |
Back to Top
Blood Diseases and Resources
Blood and Marrow Transplant Clinical Research
Network, Initiated in Fiscal Year 2001
See Chapter 11. Clinical
Trials.
Center for Human Cell Therapy, Initiated in Fiscal
Year 2004
The purpose of this Center is to serve as a unique
resource to facilitate the development of new cellular therapies for a wide
range of human diseases, especially heart, lung, and blood diseases and sleep
disorders.
Obligations
Funding History:
Fiscal Year
2005—$2,352,572
Fiscal Year 2004—$2,827,171
Total Funding to Date—$5,179,743
| Current Active
Organization and Grant Number |
- CBR Institute for Biomedical
Research
Boston, Massachusetts
|
—HL-074355 |
Functional Outcomes in Cardiovascular Patients
Undergoing Surgical Hip Fracture Repair (FOCUS), Initiated in Fiscal Year
2003
The purpose of this trial is to test whether a more
aggressive transfusion strategy that maintains postoperative Hgb levels above
10 g/dl improves functional outcome in cardiovascular patients who are over age
50 and undergoing surgical hip fracture surgery compared to a more conservative
strategy that withholds blood transfusion until the patient develops symptoms
of anemia.
Obligations
Funding History:
Fiscal Year
2005—$2,922,730
Fiscal Years
2003–2004—$3,435,202
Total Funding to Date—$6,357,932
| Current Active
Organization and Grant Number |
- Robert Wood Johnson Medical School University
of Medicine and Dentistry of New Jersey
Piscataway, New Jersey
|
—HL-073958 |
- Maryland Medical Research Institute,
Inc.
Baltimore, Maryland
|
—HL-074815 |
Genotypic Determinants of Aspirin Response in High
Risk Families, Initiated in Fiscal Year 2002
The purpose of this study is to identify genes that
may modify aspirin’s effect on platelet function and inflammatory
biomarker levels in families at increased risk for premature coronary artery
disease.
Obligations
Funding History:
Fiscal Year
2005—$3,307,112
Fiscal Years
2002–2004—$8,751,227
Total Funding to Date—$12,058,339
| Current Active
Organization and Grant Number |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-072518 |
Back to Top
Stroke With Transfusions Changing to Hydroxyurea,
Initiated in Fiscal Year 2005
The purpose of this phase III clinical trial is to
compare standard therapy (transfusions and chelation) with alternative therapy
(hydroxyurea and phlebotomy) for the prevention of secondary stroke and
management of iron overload in children with sickle cell anemia. Additional
objectives include comparisons of growth and development, frequency of
nonstroke neurological and other sickle-related events, and quality of life.
The patient population will be black.
Obligations
Funding History:
Fiscal Year
2005—$3,345,345
Total Funding to Date—$3,345,345
| Current Active
Organizations and Grant Numbers |
- St. Jude Children’s Research
Hospital
Memphis, Tennessee
|
—HL-077878 |
- Rho Federal Systems Division, Inc.
Chapel
Hill, North Carolina
|
—HL-078987 |
Thalassemia (Cooley’s Anemia) Clinical Research
Network
See Chapter 11. Clinical
Trials.
Transfusion Medicine/Hemostasis Clinical Research
Network, Initiated in Fiscal Year 2002
See Chapter 11. Clinical
Trials.
National Center on Sleep Disorders Research
Apnea Positive Pressure Long-Term Efficacy Study
(APPLES), Initiated in Fiscal Year 2002
The purpose of this study is to evaluate the
effectiveness of continuous positive airway pressure (CPAP) therapy to provide
significant, stable, and long-term neurocognitive or other benefits to patients
with obstructive sleep apnea (OSA). Investigators will identify specific
neurocognitive deficits associated with OSA and determine which ones are
reversible and most sensitive to the effects of CPAP therapy.
Obligations
Funding History:
Fiscal Year
2005—$3,188,172
Fiscal Years
2002–2004—$9,354,009
Total Funding to Date—$12,542,181
| Current Active
Organization and Grant Number |
- Stanford University
Stanford,
California
|
—HL-068060 |
Sleep Heart Health Study, Initiated in Fiscal Year
1999
The purpose of this multicenter observational study is
to determine the degree to which sleep apnea is an independent or contributing
risk factor for the development of cardiovascular or cerebrovascular
disease.
Obligations
Funding History:
Fiscal Year
2005—$1,494,336
Fiscal Years
1999–2004—$18,109,357
Total Funding to Date—$19,603,693
| Current Active
Organizations and Grant Numbers |
- University of California, Davis
Davis,
California
|
—HL-053916 |
- New York University Medical Center
New
York, New York
|
—HL-053931 |
- University of Minnesota, Twin
Cities
Minneapolis, Minnesota
|
—HL-053934 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-053937 |
- University of Arizona
Tucson, Arizona
|
—HL-053938 |
- Boston University
Boston,
Massachusetts
|
—HL-053941 |
- Missouri Breaks Research, Inc.
Timberlake,
South Dakota
|
—HL-063429 |
- Case Western Reserve University
Cleveland,
Ohio
|
—HL-063463 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-064360 |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HL-077813 |
Back to Top
NHLBI Research Centers (P50, U54, P30) Programs
Specialized Centers of Research (P50) and Specialized
Centers of Clinically Oriented Research (P50) Programs
The NHLBI initiated the Specialized Centers of
Research (SCOR) program in 1971 to encourage translational
research—converting basic science findings to the clinic—in high
priority areas. The SCOR concept emphasizes multidisciplinary research (i.e.,
basic science and clinical investigations) on diseases relevant to the
Institute’s mission. In 2002, the NHLBI revised the SCOR
program—primarily on recommendation from the NHLBAC—to place more
emphasis on clinical research projects. The newly developed SCCOR program still
requires clinical and basic scientists to work together on a unified theme, but
now requires at least 50 percent of the projects to be clinical. Listed below
is the funding history for the individual SCORs/SCCORs supported by the
Institute.
| |
Obligations
(Dollars in Thousands) |
| Area of Concentration |
Period of Operation |
Prior to FY 2005 |
FY
2005 |
Total to Date |
| Heart and
Vascular Diseases Program |
|
|
|
|
|
Cardiac Dysfunction and Disease
(SCCOR) |
2005– |
$ --- |
$16,497 |
$16,497 |
|
Molecular Genetics of
Hypertension |
1996– |
82,634 |
10,285 |
92,919 |
|
Molecular Medicine and
Atherosclerosis |
1997– |
60,235 |
8,251 |
68,486 |
|
Pediatric Heart Development and
Disease (SCCOR) |
2004– |
13,245 |
13,245 |
26,528 |
| Subtotal, Heart and
Vascular Diseases Program |
|
156,114 |
48,316 |
204,430 |
| Lung Diseases
Program |
|
|
|
|
|
Airway Biology and Pathogenesis
of Cystic Fibrosis |
1988– |
58,758 |
3,615 |
62,373 |
|
Cellular and Molecular
Mechanisms of Asthma |
1996– |
102,887 |
15,501 |
118,388 |
|
Pathobiology of Fibrotic Lung
Disease |
1997– |
39,023 |
5,428 |
44,451 |
|
Pathobiology of Lung
Development |
1996– |
62,672 |
7,585 |
70,257 |
|
Translational Research in Acute
Lung Injury (SCCOR) |
2003– |
23,326 |
12,306 |
35,632 |
| Subtotal, Lung Diseases
Program |
|
286,666 |
44,435 |
331,101 |
| Blood Diseases
and Resources Program |
|
|
|
|
|
Hemostatic and Thrombotic
Disorders |
1996– |
169,832 |
7,594 |
177,426 |
|
Transfusion Biology and
Medicine |
1996– |
62,095 |
3,267 |
65,362 |
|
Transfusion Biology and
Medicine (SCCOR) |
2005– |
--- |
4,400 |
4,400 |
|
Translational Human Stem Cell
Research |
2005– |
--- |
1,509 |
1,509 |
| Subtotal, Blood Diseases
and Resources Program |
|
231,927 |
16,770 |
248,697 |
| National Center
on Sleep Disorders Research |
|
|
|
|
|
Neurobiology of Sleep and Sleep
Apnea |
1998– |
35,102 |
6,208 |
41,310 |
| Subtotal, National
Center on Sleep Disorders Research |
|
35,102 |
6,208 |
41,310 |
| Total,
Specialized Centers of Research (P50) |
|
$709,809 |
$115,729 |
$825,532 |
Heart and Vascular Diseases Program
Cardiac Dysfunction and Disease
The purpose of this SCCOR is to foster
multidisciplinary research on clinically relevant questions related to
dysfunction and disease of the myocardium. The program will enable rapid
application of basic science findings to the prevention, diagnosis, and
treatment of cardiac disorders, including ischemic and other cardiomyopathies,
left ventricular dysfunction, metabolic abnormalities, heart failure, and
rhythm disturbances. Because some segments of the population disproportionately
suffer from heart disease, research that addresses issues of health disparity
will be emphasized.
Obligations
Fiscal Year 2005—$16,497,136
| Current Active
Organizations and Grant Numbers |
- Columbia University Health Science
Center
New York, New York
|
—HL-077096 |
- University of Alabama at
Birmingham
Birmingham, Alabama
|
—HL-077100 |
- University of Cincinnati
Cincinnati,
Ohio
|
—HL-077101 |
- Cleveland Clinical Lerner
College
Cleveland, Ohio
|
—HL-077107 |
- Washington University
St. Louis,
Missouri
|
—HL-077113 |
Molecular Genetics of Hypertension
The purpose of this SCOR is to elucidate the etiology
and pathogenesis of hypertension and to translate the knowledge into improved
diagnosis and management of the disease.
Obligations
Fiscal Year 2005—$10,285,182
| Current Active
Organizations and Grant Numbers |
- Medical College of Wisconsin
Milwaukee,
Wisconsin
|
—HL-054998 |
- Brigham and Women’s Hospital
Boston,
Massachusetts
|
—HL-055000 |
- Boston University Medical Center
Boston,
Massachusetts
|
—HL-055001 |
- University of Iowa
Iowa City, Iowa
|
—HL-055006 |
- Yale University
New Haven,
Connecticut
|
—HL-055007 |
Molecular Medicine and Atherosclerosis
The goal of this SCOR is to advance understanding of
the etiology and pathobiology of the atherosclerotic lesion at the molecular
level through modern methods and approaches of molecular medicine. Some of the
subprojects have a large minority patient population.
Obligations
Fiscal Year 2005—$8,251,334
| Current Active
Organizations and Grant Numbers |
- Columbia University
New York, New
York
|
—HL-056984 |
- Brigham and Women’s Hospital
Boston,
Massachusetts
|
—HL-056985 |
- University of California, San Diego
La
Jolla, California
|
—HL-056989 |
- University of Pennsylvania
Philadelphia,
Pennsylvania
|
—HL-070128 |
Pediatric Heart Development and Disease
The purpose of this SCCOR is to foster
multidisciplinary collaborations so that basic research advances can be
translated rapidly to clinical care for children with heart disease. Research
focus ranges from the genetic basis of heart valve disease to clinical trials
of novel surgical strategies for congenital heart disease repair and immune
modulation in pediatric heart transplantation. Two of the centers will have
Clinical Research Skills Development Cores to train fellows and junior faculty
in clinical research methods.
Obligations
Fiscal Year 2005—$13,282,825 Fiscal Year
2005—$3,615,257
Lung Diseases Program
Airway Biology and Pathogenesis of Cystic
Fibrosis
The goals of this SCOR are to investigate the basic
mechanisms underlying cystic fibrosis, develop new hypotheses, and apply
innovative strategies for approaching clinical and fundamental issues.
Obligations
Fiscal Year 2005—$3,615,257
| Current Active
Organizations and Grant Numbers |
- University of North Carolina at Chapel
Hill
Chapel Hill, North Carolina
|
—HL-060280 |
- University of Iowa
Iowa City, Iowa
|
—HL-061234 |
Cellular and Molecular Mechanisms of Asthma
The objective of this SCOR is to apply critical
science and technology to increase understanding of cellular and molecular
mechanisms of asthma, including those mechanisms underlying the biological
impact of environmental factors.
Obligations
Fiscal Year 2005—$15,501,156
| Current Active
Organizations and Grant Numbers |
- University of New Mexico
Albuquerque, New
Mexico
|
—HL-056384 |
- University of California, San
Francisco
San Francisco, California
|
—HL-056385 |
- University of Wisconsin
Madison,
Wisconsin
|
—HL-056396 |
- University of Chicago
Chicago,
Illinois
|
—HL-056399 |
- Washington University
St. Louis,
Missouri
|
—HL-056419 |
- University of Pennsylvania
Philadelphia,
Pennsylvania
|
—HL-067663 |
- Beth Israel Deaconess Medical
Center
Boston, Massachusetts
|
—HL-067664 |
- University of Arizona
Tucson, Arizona
|
—HL-067672 |
- Stanford University
Stanford,
California
|
—HL-067674 |
Pathobiology of Fibrotic Lung Disease
The purpose of this SCOR is to study cellular and
molecular mechanisms involved in transition from inflammatory events associated
with early fibrotic disease to later processes involving wound healing, repair,
and fibrosis.
Obligations
Fiscal Year 2005—$5,427,833
| Current Active
Organizations and Grant Numbers |
- University of Michigan at Ann Arbor
Ann
Arbor, Michigan
|
—HL-056402 |
- University of California, Los Angeles
Los
Angeles, California
|
—HL-067665 |
- National Jewish Center for Immunology and
Respiratory Diseases
Denver, Colorado
|
—HL-067671 |
Pathobiology of Lung Development
The objective of this SCOR is to foster
multidisciplinary research enabling basic science findings to be rapidly
applied to clinical problems related to lung development. The program focuses
on identification of the molecular variables involved in lung development and
assessment of the impact of injury during critical periods.
Obligations
Fiscal Year 2005—$7,584,895
| Current Active
Organizations and Grant Numbers |
- Children’s Hospital Medical
Center
Cincinnati, Ohio
|
—HL-056387 |
- Children’s Hospital of
Philadelphia
Philadelphia, Pennsylvania
|
—HL-056401 |
- University of Colorado Health Sciences
Center
Denver, Colorado
|
—HL-057144 |
- Children’s Hospital
Boston,
Massachusetts
|
—HL-067669 |
Translational Research in Acute Lung Injury
The purpose of this SCCOR is to foster
multidisciplinary research to improve the prevention, diagnosis, and treatment
of acute lung injury and its more severe form—adult respiratory distress
syndrome. This program includes phase II clinical trials and studies of
molecular mechanisms of inflammation and coagulation, gene and protein
expression, and cell and animal models of lung injury.
Obligations
Fiscal Year 2005—$12,305,723
| Current Active
Organizations and Grant Numbers |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-073994 |
- University of Washington
Seattle,
Washington
|
—HL-073996 |
- University of California, San
Francisco
San Francisco, California
|
—HL-074005 |
- University of Michigan at Ann Arbor
Ann
Arbor, Michigan
|
—HL-074024 |
Blood Diseases and Resources Program
Hemostatic and Thrombotic Disorders
The purpose of this SCOR is to investigate pathogenic
mechanisms involved in human thrombotic disease and to develop improved methods
for its diagnosis and treatment. One of the studies has a large minority
patient population.
Obligations
Fiscal Year 2005—$7,543,875
| Current Active
Organizations and Grant Numbers |
- Mount Sinai School of Medicine
New York,
New York
|
—HL-054469 |
- University of Pennsylvania
Philadelphia,
Pennsylvania
|
—HL-054500 |
- University of Oklahoma
Oklahoma City,
Oklahoma
|
—HL-054502 |
- Baylor College of Medicine
Houston,
Texas
|
—HL-065967 |
Transfusion Biology and Medicine
The purpose of this SCOR is to foster new approaches
for improving the availability, efficacy, safety, and quality of blood and
blood products for therapeutic uses. One of the centers has a large minority
population.
Obligations
Fiscal Year 2005—$3,267,069
| Current Active
Organizations and Grant Numbers |
- New York Blood Center
New York, New
York
|
—HL-054459 |
- University of California, San
Francisco
San Francisco, California
|
—HL-054476 |
Transfusion Biology and Medicine
The purpose of this SCCOR is to foster
multidisciplinary research on clinically relevant questions to enable rapid
application of basic science findings to clinical problems associated with
improving the availability, efficacy, safety, and quality of blood and blood
products for therapeutic uses.
Obligations
Fiscal Year 2005—$4,400,002
| Current Active
Organizations and Grant Numbers |
- Puget Sound Blood Center
Seattle,
Washington
|
—HL-081015 |
- University of California, San
Francisco
San Francisco, California
|
—HL-081027 |
Translational Human Stem Cell Research
The purpose of this SCCOR is to stimulate
multi-disciplinary collaboration among basic stem cell biologists, researchers,
and clinicians with disease-specific expertise, physicians and surgeons skilled
in innovative modes of cell delivery, and investigators experienced in
developing and assessing animal models of human diseases to conduct
hitherto-unexplored projects such as preclinical studies for cell-based therapy
employing human stem cells in animal models. Research findings will ultimately
lead to innovative approaches for the prevention, treatment, and cure of
disease, and will accelerate the translation of basic scientific discoveries
into new therapies.
Obligations
Fiscal Year 2005—$1,509,262
| Current Active
Organizations and Grant Numbers |
- University of California, Davis
Davis,
California
|
—HL-085036 |
National Center on Sleep Disorders Research
Neurobiology of Sleep and Sleep Apnea
The objective of this SCOR is to integrate molecular,
cellular, and genetic approaches to sleep control with clinical investigations
on the etiology and pathogenesis of sleep disorders, particularly sleep
apnea.
Obligations
Fiscal Year 2005—$6,208,020
| Current Active
Organizations and Grant Numbers |
- University of Pennsylvania
Philadelphia,
Pennsylvania
|
—HL-060287 |
- Brigham and Women’s Hospital
Boston,
Massachusetts
|
—HL-060292 |
- University of California, Los Angeles
Los
Angeles, California
|
—HL-060296 |
Comprehensive Sickle Cell Centers (U54) Program
The Comprehensive Sickle Cell Centers (CSCC) were
instituted in FY 1972 to bridge the gap between research and service by
combining basic and clinical research, clinical trials and applications
training, and community service projects into one program. The patients
recruited for the clinical studies are primarily from minority populations.
Obligations
Fiscal Year 2005—$24,313,796
| Current Active
Organizations and Grant Numbers |
- Children’s Hospital and Research
Center
Oakland, California
|
—HL-070583 |
- Thomas Jefferson University
Philadelphia,
Pennsylvania
|
—HL-070585 |
- Rho Federal Systems Division, Inc.
Chapel
Hill, North Carolina
|
—HL-070587 |
- University of Texas Southwestern Medical
Center
Dallas, Texas
|
—HL-070588 |
- St. Jude Children’s Research
Hospital
Memphis, Tennessee
|
—HL-070590 |
- University of Southern California
Los
Angeles, California
|
—HL-070595 |
- Children’s Hospital of
Philadelphia
Philadelphia, Pennsylvania
|
—HL-070596 |
- Duke University
Durham, North
Carolina
|
—HL-070769 |
- Boston Medical Center
Boston,
Massachusetts
|
—HL-070819 |
- Children’s Hospital Research
Center
Cincinnati, Ohio
|
—HL-070871 |
- Yeshiva University
New York, New York
|
—HL-070994 |
Specialized Centers for Cell-Based Therapies for
Heart, Lung, and Blood Diseases (U54) Program
The Specialized Centers for Cell-Based Therapies
Program, which includes a Data and Coordinating Center, was initiated in FY
2005 to support preclinical and clinical studies for cell-based therapy for
heart, lung, and blood diseases and sleep disorders. A key feature of the
program is the ability to conduct preclinical studies in the first year or two
of the program, in order to meet the requirements for an Investigational New
Drug application prior to initiating clinical studies. Clinical studies are
expected to be initiated by the beginning of the third year.
Obligations
Fiscal Year 2005—$6,957,161
| Current Active
Organizations and Grant Numbers |
- Baylor College of Medicine
Houston,
Texas
|
—HL-081007 |
- EMMES Corporation
Rockville, Maryland
|
—HL-081021 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HL-081028 |
- Massachusetts General Hospital
Boston,
Massachusetts
|
—HL-081030 |
Centers for AIDS Research (P30) Program
The NHLBI, along with five other NIH Institutes,
contributes to the support of six Centers for AIDS Research that were
established to provide a multidisciplinary environment that promotes basic,
clinical, behavioral, and translational research activities in the prevention,
detection, and treatment of HIV infection and AIDS. Almost half of the patient
population comes from minority groups.
Obligations
Fiscal Year 2005—$2,829,119
| Current Active
Organizations and Grant Numbers |
- New York University School of Medicine
New
York, New York
|
—AI-27742 |
- University of Washington
Seattle,
Washington
|
—AI-27757 |
- University of California, San
Francisco
San Francisco, California
|
—AI-27763 |
- University of Alabama at
Birmingham
Birmingham, Alabama
|
—AI-27767 |
- University of California, Los Angeles
Los
Angeles, California
|
—AI-28697 |
- Baylor University
Houston, Texas
|
—AI-36211 |
- University of California, San Diego
La
Jolla, California
|
—AI-36214 |
- Case Western Reserve University
Cleveland,
Ohio
|
—AI-36219 |
- University of Massachusetts Medical
School
Worcester, Massachusetts
|
—AI-42845 |
- Miriam Hospital
Providence, Rhode
Island
|
—AI-42853 |
- Johns Hopkins University
Baltimore,
Maryland
|
—AI-42855 |
- University of Pennsylvania
Philadelphia,
Pennsylvania
|
—AI-45008 |
- Emory University
Atlanta, Georgia
|
—AI-50409 |
- University of North Carolina at Chapel
Hill
Chapel Hill, North Carolina
|
—AI-50410 |
- Yeshiva University
New York, New York
|
—AI-51519 |
- University of Colorado Health Sciences
Center
Denver, Colorado
|
—AI-54907 |
- Vanderbilt University
Nashville,
Tennessee
|
—AI-54999 |
- Harvard Medical School
Boston,
Massachusetts
|
—AI-60354 |
- Duke University
Durham, North
Carolina
|
—AI-64518 |
Back to Top
« Factbook Table of
Contents |
