 |
Incorrect Product Claim Ad
A product claim ad names a drug, says what condition it treats, and talks about both its benefits and its risks. An ad must present the benefits and risks of a prescription drug in a balanced fashion. Balance depends on both the information in the ad itself and how the information is presented.
There are 4 major problems with this ad:
- The ad misleadingly suggests that Arbitraer is approved to treat children by showing an image of a young girl. Remember that these ads must suggest only uses that have been approved by FDA.
- The ad does not present a "fair balance" of information about the drug's risks compared with its benefits because the ad minimizes the important risks of Arbitraer. The risks appear in much smaller type than the benefits and are placed in a corner of the ad far from the benefits and are likely to be overlooked.
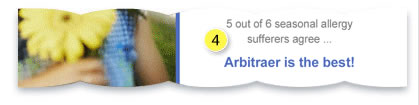
- The ad makes false and misleading claims about Arbitraer. It is not approved to treat asthma symptoms. Also, no studies support the claims that Arbitraer works "in no time," and that most allergy sufferers think Arbitraer is "the best." All ad information needs to be supported by well-designed studies.
- The ad does not contain the required "brief summary" of information that includes all the risks listed in the FDA-approved prescribing information.
Choose a yellow number in the ad for detailed information.
View a Correct Product Claim Ad |

|
|
 |
The image of the young girl in the ad is misleading because the fictional drug is approved for use only in adults 18 years of age and older.
Back to top |
 |
|
 |
Although claims generally must be supported by data from well-designed studies, consumers may not know if such studies exist or what they show. If FDA determines that claims are not supported, it will take action to have the ad fixed. In the short term, if you have doubts about a claim in an advertisement, you should talk to your healthcare provider.
Back to top |
 |
|
 |
This ad falsely states that Arbitraer is approved to help control asthma symptoms. This fictional drug (see the Correct Product Claim Ad) is approved to treat seasonal nasal allergy symptoms.
Back to top |
 |
|
 |
As stated above, although claims generally must be supported by data from well-designed studies, consumers may not know if such studies exist or what they show. If FDA determines that claims are not supported, it will take action to have the ad fixed. In the short term, if you have doubts about a claim in an advertisement, you should talk to your healthcare provider.
Back to top |
 |
|
 |
This ad presents Arbitraer's risks in small type size and positions this information far from where the benefits are discussed, so it is harder for the reader to notice and read the risks. "Fair balance" requires that risks and benefits be similarly clear.
Back to top |
 |
|
 |
The ad does not include the "brief summary," which includes additional required risk information. The law requires that ads include this "brief summary." Also, the ad does not include the statement "You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088." This statement is required to be included in print ads by the Food and Drug Amendments Act of 2007.
Back to top |
 |
 Back to Top
Back to Top
 Back to Prescription Drug Ads
Back to Prescription Drug Ads
 PDF requires the free
Adobe Acrobat Reader PDF requires the free
Adobe Acrobat Reader
Date created: September 3, 2008 |
 |