

 |
 |
Document issued on: June 15, 2006
SUBJECT: INSPECTION OF MEDICAL DEVICE MANUFACTURERS
IMPLEMENTATION DATE: June 15, 2006 (Previous editions obsolete.)
COMPLETION DATE: June 15, 2010
DATA REPORTING
PRODUCT CODES: 73-91
PRODUCT/ASSIGNMENT CODES:
82845A; 42845A -- All Level 1 (Abbreviated) Inspections
82845B; 42845B -- All Level 2 (Comprehensive) Inspections
82845C; 42845C -- All Level 3 (Compliance Follow-up) Inspections
82845G -- All For Cause Inspections
82845P -- Joint FDA/Accredited Person Inspections
82845S -- Report Time spent on Assessment of Firm’s Sterilization processes
81010 -- Report Time spent on MDR Follow-up
81011 -- Report Time spent on Assessment of Firm’s MDR Practices
81845T -- Report Time spent on Assessment of Firm’s Tracking Practices
81845R -- Report Time spent on Assessment of Firm’s Corrections and Removals Practices
82A800 -- Independent Accredited Person Inspections
Coversheet: Field Reporting Requirements
Part V: Regulatory/Administrative Follow-up
Part VI: References and Program Contacts
Attachments
Attachment A: CDRH Office of Compliance Organizational Chart
Attachment B: CDRH Office of In Vitro Diagnostic Devices Organizational Chart
Attachment C: Summary of MDR Reporting Requirements
Attachment D: Summary of Tracking Requirements
Attachment E: Summary of Corrections and Removals Requirements
EIRs: All recommendations for administrative/regulatory action should include the EIR, FDA-483, and exhibits. The recommendations should be sent to the Center for Devices and Radiological Health (CDRH) HFZ-306 and for human cells, tissues, and cellular and tissue-based products (HCT/Ps), or combination products the recommendations should also be sent to the Center for Biologics Evaluation and Research (CBER) and/or the Center for Drug Evaluation and Research (CDER) as appropriate.
Warning Letters: A copy of all Warning Letters related to all requirements covered in this compliance program should be sent to HFZ-306 and HFC-210.
Comment:
This guidance document represents the agency’s current thinking on the enforcement of the Quality System (QS), Medical Device Reporting (MDR), Medical Device Tracking, Corrections and Removals, and the Registration and Listing regulations. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. An alternative approach may be used if such approach satisfies the requirements of the applicable statute, regulations, or both.
PROGRAM |
PACs |
Quality System
|
Level 1 (82845A) |
Level 2 (82845B) |
|
Level 3 (82845C) |
|
For Cause |
82845G |
Joint FDA/Accredited Persons |
82845P |
Independent Accredited Person Inspection |
82A800 |
MDR |
81010 & 81011 |
Tracking |
81845T |
CAR |
81845R |
Sterilization Inspections |
82845S |
Note: When conducting sterilization review as part of the Production and Process Controls subsystem, report only the time spent reviewing the sterilization process during the Quality System inspection, if covered under PAC 82845S. Also, report PACs, 81010, 81011, 81845T and 81845R, as applicable.
The above PAC Guidance is provided for investigator reference only. Additional CBER and/or CDER PAC codes may also by necessary for multi-jurisdictional products (i.e. tissue) and combination products. Please refer to the inspection assignment for guidance
This compliance program provides guidance to FDA field and center staffs for the inspections and administrative/enforcement activities related to the Quality System (QS) regulation (21 CFR Part 820), the Medical Device Reporting (MDR) regulation (21 CFR Part 803), the Medical Device Tracking regulation (21 CFR Part 821), the Corrections and Remov als regulation (21 CFR Part 806), and the Registration and Listing regulation (21 CFR Part 807). This compliance program supersedes the program of the same name which was issued on October 1, 2000.
This compliance program encom passes five regulations for inspecting medical device firms. Under the QS regulation, manufacturers are expected to control their devices from design stage through post-market surveillance. Manufacturing processes, such as sterilization, are required to be implemented under appropriate controls. The MDR, Tracking, and Corrections and Removals regulations involve activities with which manufacturers and importers are required to comply after the devices are distributed. This compliance program provides specific guidance for each. It also requires coverage for the Registration & Listing regulation.
Manufacturers establish and follow quality systems to help ensure that their products consistently meet applicable requirements and specifications. The quality systems for FDA-regulated products (food, drugs, biologics, and devices) are known as CGMP's. CGMP requirements for devices in part 820 (21 CFR part 820) were first authorized by section 520(f) of the Federal Food, Drug, and Cosmetic Act (the act) (21 U.S.C. 360j(f)), which was among the authorities added to the act by the Medical Device Amendments of 1976. Under section 520(f) of the act, FDA issued a final rule in the Federal Register of July 21, 1978 (43 FR 31 508), prescribing CGMP requirements for the methods used in, and the facilities and controls used for the manufacture, packing, storage, and installation of medical devices. This regulation became effective on December 18, 1978.
The Safe Medical Devices Act of 1990 (the SMDA), enacted on November 28, 1990, amended section 520(f) of the act, providing FDA with the authority to add preproduction design controls to the CGMP regulation. This change in law was based on findings that a significant proportion of device recalls were attributed to faulty design of product. The SMDA also added new section 803 to the act (21 U.S.C. 383) which, among other things, encourages FDA to work with foreign countries toward mutual recognition of CGMP requirements. FDA undertook the revision of the CGMP regulation to add the design controls authorized by the SMDA to the CGMP regulation, as well as because the agency believed that it would be beneficial to the public and the medical device industry for the CGMP regulation to be consistent, to the extent possible, with the requirements for quality systems contained in applicable international standards. FDA published the revised CGMP requirements in the final rule entitled “Quality System Regulation” in the Federal Register of October 7, 1996 (61 FR 52602). This regulation became effective on June 1, 1997 and remains in effect.The first Medical Device Reporting (MDR) regulation became final on December 13, 1984. As a result of changes mandated by the Safe Medical Devices Act (SMDA) of 1990, and the Medical Device Amendments of 1992, the 1984 MDR regulations (21 CFR 803 & 807) were revised and published on December 11, 1995. The FDA Modernization Act of 1997 made additional changes and a revised MDR regulation was proposed in May 1998. The final revised MDR regulation was published in the Federal Register on January 26, 2000. The latest version of MDR regulation includes reporting requirements for manufacturers, user facilities, and importers. MDR reporting for medical device distributors (except importers) was revoked by the FDA Modernization Act of 1997. Distributors are, however, still required to maintain complaint records, per 21 CFR 803.18(d)(1-3).
21 CFR Part 803 requires manufacturers of medical devices, including in vitro diagnostic devices, to report to FDA whenever the manufacturer or importer receives or otherwise becomes aware of information that reasonably suggests that one of its marketed devices:
NOTE: Importers (initial distributors) of medical devices are subject to 21 CFR Part 803 published in the Federal Register on January 26, 2000, and effective March 27, 2000.
Under the authority of section 519(e) of the Act, the agency may issue a written tracking “order” that tells a manufacturer to implement a tracking program that meets the requirements of 21 CFR Part 821. Devices subject to tracking may include those that are permanently implanted or life sustaining/life supporting devices that are used outside a device user facility. These devices are considered reasonably likely to cause serious adverse health consequences if they fail. The regulation is intended to ensure that in the event of a recall or safety alert, a tracked device can be traced by the manufacturer from the device manufacturing facility to the end user or patient.
The Corrections and Removal regulation requires manufacturers, and importers to report promptly to FDA any corrections or removals of devices being undertaken to reduce risk to health.
The Registration and Listing regulation requires manufacturers and foreign exporters to register and list their devices; and importers to register. (See Part III)
B. PROGRAM MANAGEMENT INSTRUCTIONS
- This compliance program is to be used to conduct Quality System inspections of devices. The profile information should be updated in FACTS for QS inspections. Instructions for updating firm profiles in FACTS are referenced in the IOM Exhibit 5-13, and on the Office of Enforcement’s intranet.
- Many large firms have several manufacturing facilities located in more than one district. These firms often have a research and development (R&D) center or corporate design facility, which services several manufacturing facilities.
- Upon completing an inspection of an R&D center or corporate design facility, districts should send copies of the inspection report to the home districts of the firm’s manufacturing facilities.
- Unless additional information must be obtained from the manufacturing facility, the home district of the manufacturing facility during the next inspection need only verify the coordination aspects of the design control activities as long as the inspection of the R&D center or corporate design facility was conducted within the previous two years. Examples of design control coordination activities are:
- How design change information is shared, verified, and, where appropriate, validated as full scale manufacturing;
- How design transfer activities at the manufacturing facility are verified;
- How the risk analysis is performed with respect to manufacturing controls; and,
- How the risk analysis is continually being updated as manufacturing changes occur.
- Likewise, if an inspection of the R&D center or corporate design facility has not been conducted within the previous two years, the home district of the manufacturing facility should issue an assignment to the home district of the R&D center or corporate design facility requesting a design control inspection. The above guidance is NOT applicable to Pre-Approval inspections.
- c. Sterilization of medical devices is covered as a part of the QSIT inspection under this compliance program. Guidance provided in the QSIT Guide is to be followed when inspecting sterilization processes for the following types of facilities:
Medical Devices related to AIDS diagnosis and screening, blood banking, blood screening and/or human blood processing will be inspected under this compliance program and CBER’s compliance program 7342.008, “Inspection of Licensed Viral Marker Test Kits.” For guidance, see the Intercenter Agreement between the Center for Biologics Evaluation and Research, and the Center for Devices and Radiological Health, dated October 31, 1991. The Biologics and Devices Intercenter Agreement can be found at the following web site: http://www.fda.gov/oc/omb udsman/bio-dev.htm
- Class I Device Manufacturers
All Class I devices, including those exempted from most of the Quality System regulation requirements, must comply with record keeping requirements and complaint file requirements, as well as reporting requirements under the MDR regulation. Class I manufacturers should not be routinely scheduled for inspection but should receive lowest inspectional priority unless addressed by a special, “For Cause” assignment or when a health hazard is ap parent. Use the following link to determine if a device is Class I exempt from QS requirements. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPCD/classification.cfm
If inspecting a manufacturer that was originally planned as a Class I QS non-exempt, Class II or III device firm, and the inspection finds that the firm no longer makes Class I QS non-exempt, Class II or Class III devices, the investigator should review the firm's complaint handling system and MDR practices, then terminate the inspection. The District should report the time against PAC 82845A.
This program includes guidance for determining compliance with the Quality System (QS) regulation, Medical Device Reporting (MDR) regulation, Medical Device Tracking regulation, Corrections and Removals regulation, and the Registration and Listing regulation.
1. Inspectional Strategy
The QS inspectional goal is to assess the firm’s quality management system for compliance with the appropriate regulations. The QS inspections should generally start with a walk through of the facility to become familiar with the firm’s operations and general state of control. See IOM 5.1.2.2.
The inspection will assess the firm’s systems, methods, and procedures to ensure that the firm’s quality management system is effectively established (defined, documented and implemented) and effectively maintained. QS inspections should include the assessment of post-market information on distributed devices to include:
- Review of recalls
- Review of MDRs (Be alert to the fact that MDRs may contain information on recalls that have not been reported through the district under 21 CFR Part 806.)
- Review of corrections and removals
- Review of significant changes in device specifications or in the manufacturing specifications
- Follow-up on previous FDA 483 observation(s), to include the corrections, corrective actions or preventive actions for the observation(s) and the related system(s)
Available post-market information should be reviewed as a part of the preparation for the inspection, in order to facilitate efficient time spent at the facility. Identify in the EIR post-market information reviewed during the inspection and adequately document your findings. See IOM 5.10.4.3.9. Any problems identified as a result of the review of post-market information should be developed during the inspection.
Important Note : The review of post-market information does not mean that the investigator should open the inspection with the review of complaints and complaint information. Complaints should be reviewed within the context of the Corrective and Preventive Action sub-system according to the procedures described below in this part.
a. QS Inspections
QS inspections should generally be conducted using the Quality System Inspection Technique (QSIT). Guidance for performing an inspection is provided in the Guide to Inspections of Quality Systems, August 1999, also called the QSIT Guide www.fda.gov/ora/inspect_ref/igs/qsit/qsitguide.htm. This QSIT tool can be scaled to meet the needs of each particular inspection. The table below correlates the level of inspection and the guidance on how to perform the inspections.
Inspection
LevelType of
InspectionGuide to Inspections
1
Abbreviated
QSIT – Two subsystems; Corrective and Preventive Actions (CAPA) plus Production and Process Controls (P&PC) or Design Controls
(PAC 82845A)
2
Comprehensive
QSIT - The four major subsystems; Management Controls, Design Controls, CAPA and P&PC
(PAC 82845B or 82845P or 82A800)
3
Compliance Follow-up*
As directed by inspectional guidance and elements of QSIT
(PAC 82845C)
Special
For Cause*
As directed by inspectional guidance and elements of QSIT
(PAC 82845G)
* Compliance Follow-up and For Cause inspections are dictated by the previous FDA 483 findings and other regulatory information and may differ from the typical QSIT approach. The inspectional guidance provided by the assignment, the district compliance branch, and/or CDRH will guide the direction of these inspections. However, elements of the QSIT Guide may also be utilized. See further details below. Investigators must ensure that the EIR clearly states what was covered during the inspection due to the directed nature of these types of inspections.
NOTE: The Quality System regulation can be grouped into seven subsystems; however, the following four subsystems are considered major subsystems and are the basic foundation of a firm’s quality management system: Management Controls, Design Controls, Corrective and Preventive Actions (CAPA), and Production and Process Controls (P&PC). MDR, Corrections and Removals, and Tracking requirements (where applicable) should be covered when covering the CAPA subsystem. The three remaining subsystems (Facilities and Equipment Controls, Materials Controls and Document/Records/Change Controls) cut across a firm’s quality management system and are evaluated while covering the four major subsystems.
In the work plan, Level 1 Abbreviated (82845A), Level 2 Comprehensive (82845B), Level 3 Compliance Follow-Up (82845C), For Cause (82845G), and Accredited Persons (82845P or 82A800) inspections are planned for each district. Planning resources for these five PACs provides greater control, at the district level, on the type of inspection conducted to maximize resource utilization and provide the flexibility needed to insure the Performance Goals are met. In utilizing this flexibility, districts must continue to monitor their accomplishments to assure that the Performance Goals and work plan are met.
b. Level 1 Inspections - PAC 82845A
Level 1 inspections are Abbreviated Inspections.
This level of inspection (CAPA plus P&PC or Design Controls) may be used for routine surveillance and initial inspections of all firms, other than firms that manufacture Class III devices. However, it is recommended that initial inspections of Class II manufacturers utilize a Level 2 Comprehensive inspection whenever district resources permit. Level 1 inspections should cover the CAPA subsystem, then P&PC or Design Controls, using the QSIT Guide. The selection of CAPA plus either the P&PC or Design Controls subsystem will provide an adequate review of the compliance status of the firm.
The following should be considered in determining whether to select P&PC or Design Controls:
- CAPA findings during the inspection;
- Subsystems covered during the previous EI. The previous EIR(s) should be reviewed to determine which subsystems were previously covered. The selection of the P&PC or Design Controls subsystem should be alternated over time so that more subsystems within a firm’s overall quality management system are assessed;
- Significant changes since the previous EI. Determine if there were any design changes which required a new submission or application, or if there were any major process changes; and,
- Post market information indicating potential design problems.
The EIR must clearly state which subsystem P&PC or Design Controls was chosen and why.
Note: The adequacy of the correction(s), corrective action(s) or preventive action(s) related to any FDA 483 item(s) from the previous inspection should be covered, even if the entire subsystem will not be reviewed during the current Level 1 inspection.
c. Level 2 Inspections - PAC 82845B or 82845P
Level 2 inspections are Comprehensive Inspections.
Level 2 inspections will cover all four major subsystems (Management Controls, Design Controls, CAPA, and P&PC) as explained in the QSIT Guide. The Level 2 inspection is considered a comprehensive review of the compliance status of the firm.
Level 2 inspections will be performed:
- For all initial inspections of Class III device manufacturers and where possible Class II device manufacturers
- By assignment
- For foreign inspections
- For training
- For Accredited Persons audits (PAC 82845P)
- When an inspection, which started out as Level 1, reveals post market information and/or objectionable conditions which cannot be adequately assessed as a Level 1 inspection. (Before converting to this more comprehensive level, district management should be informed.)
- Where district work plan resources permit (Level 2 should be considered for any inspections of Class II and Class III device manufacturers. The decision to use Level 2 inspections should be based on risk.)
Note: For more information on the Accredited Person audits see “Accredited Person Inspection Program (Medical Devices) Performance Audit Procedures” on the Division of Human Resource Development’s (DHRD’s) intranet website under the certification/related programs/accredited person program section.
The Level 2 QSIT approach was validated using the following inspectional sequence: Management Controls, Design Controls, CAPA and P&PC. This inspectional sequence allows the investigator to review design control issues and how the device specifications were established before reviewing the CAPA subsystem. Investigators may however start with Management Controls, followed by CAPA, Design Controls, and P&PC with appropriate linkages. Information from Design Controls and CAPA may be used to select the products and processes for inspecting production and process controls, and appropriate linkages. The subsystems may be inspected in any appropriate and justifiable sequence in order to perform a timely and effective inspection.
Selection of manufacturing processes for inspectional coverage should include the following considerations:
- CAPA indicators of process problems
- Processes used to manufacture high risk products
- Processes that have a high risk of causing product failure
- Processes that require process validation
- Processes that are new to the manufacturer
- Processes that cover a variety of process technologies and profile classes
- Common processes used in multiple products
- Processes not covered during previous inspections
It is important to thoroughly cover Purchasing Controls, to include outsourced processes, as a QSIT linkage under P&PC whenever P & PC is covered. The Purchasing Control coverage must be documented in the EIR especially if the manufacturer contracts a sterilization process or contracts the manufacture of significant components, subassemblies, or processes.
d. Level 3 Inspections - PAC 82845C
Level 3 inspections are Compliance Follow-up Inspections.
Level 3 inspections are necessary after a firm was found to have Situation I conditions during a previous QS inspection which was classified Official Action Indicated (OAI). (See Part V of this compliance program for information on Situation I and OAI.) Level 3 inspections will also be performed when directed by assignment.
The QSIT Guide should be used for guidance, but the inspectional guidance provided by the assignment, the district compliance branch, and/or CDRH will guide the flow of the inspection. The district compliance officers should be contacted during Level 3 inspections to assure that:
- Appropriate inspectional areas are covered with enough depth to support any findings
- Noncompliant findings (conditions) are adequately developed and documented
- Sufficient evidence is collected to support an appropriate regulatory action recommendation
Note: Foreign inspections, as discussed below, are Level 2 inspections and therefore the option to stop an inspection in the next two diagrams does not apply.
If the previous inspection was a Level 2 inspection:
During domestic Level 3 inspections:
(A) Verify that adequate correction(s) and corrective action(s) have been implemented to the quality system problems previously identified.
(B) If the correction(s) and corrective action(s) were not implemented or were not implemented effectively, verify that the deficiencies continue to exist and provide adequate evidence to support a possible regulatory action.
(C) Document any additional quality system problems observed during the inspection, and provide adequate evidence to support a possible regulatory action.
The chart below describes the steps for the Level 3 domestic inspection after a Level 2 inspection.
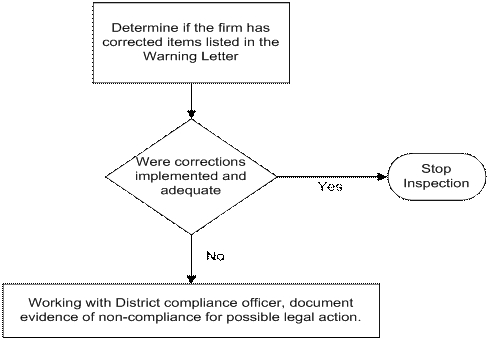
If the previous inspection was a Level 1 inspection:
When the previous inspection was performed as a Level 1 inspection, the other two major subsystems previously not covered must be covered in addition to the inspectional guidance. It is important that the combination of the Level 1 and Level 3 inspections cover all four of the major subsystems in order to ensure a comprehensive review of the firm’s quality management system.
During domestic Level 3 inspections:
(A) Verify that adequate correction(s) and corrective action(s) have been implemented to the quality system problems previously identified; and
(B) If the correction(s) and corrective action(s) were not implemented or were not implemented effectively, verify that the deficiencies continue to exist and provide adequate evidence to support a possible regulatory action.
(C) Document any additional quality system problems observed during the inspection, and provide adequate evidence to support a possible regulatory action.
The chart below describes the steps for the Level 3 domestic inspection after a Level 1 inspection.
e. For Cause Inspections - PAC 82845G
For Cause inspections are carried out in response to specific information that raises questions, concerns, or problems associated with a FDA regulated firm or commodity. This information could come to the attention of FDA from any source and including but not limited to, the following:
- Results of a sample analysis;
- Observations made during prior inspections;
- Recall or market withdrawal;
- Consumer or employee complaint;
- Adverse reaction report; or,
- Suspicion of fraud.
For Cause inspections are usually initiated at the request of CDRH, ORA headquarters, Regional or District directives. For Cause inspections are dictated by the source of information and may differ from the typical QSIT approach. These inspections are generally more in-depth in particular areas than typical QSIT inspections. The inspectional guidance provided by the assignment, the district compliance branch, and/or CDRH will guide the flow of these inspections, however, elements of the QSIT Guide may also be utilized.
For Cause inspections should be directed towards the quality problem(s), and if applicable, trace the underlying cause, assuring that appropriate correction(s) and corrective action(s) are initiated.
If a serious public health risk is encountered during a QSIT inspection, consideration should be given to performing a For Cause inspection. The district compliance branch should be consulted prior to this decision.
For Cause inspections may also be initiated at a contract sterilizer when an inspection at a device manufacturer raises questions about the adequacy of processing or quality assurance by the contract sterilizer. Likewise, an inspection at a contract sterilizer may lead to a For Cause inspection of device manufacturers if significant deficiencies are observed. The deficiencies may be an indication that the device manufacturer(s) has not assumed appropriate responsibility for the sterilization validation and processing of its own devices. The district that has identified the need for the additional coverage is to notify the home district of the establishment that needs a For Cause inspection.
f. Foreign Inspections
All foreign inspections should be conducted using the QSIT Guide under the Level 2 strategy, and any special instructions contained in the inspection assignment. The foreign manufacturer's compliance with registration and listing requirements should be covered during foreign inspections. The failure of foreign device manufacturers to list products exported to the US will subject medical devices to detention upon entry.
Foreign inspections are subject to time constraints but need to follow the instructions for a Level 2 inspection as described above. Requests for documents should be made as early as possible to give the firm time for written or oral translations and obtaining documents that may be located in US offices. Oral translations need to be documented in the EIR if that information is utilized in supporting an observation(s).
2. Inspectional Instructions
a. Required Statement(s)
The following statement should be included on each FDA 483:
This document lists observations made by the FDA representative(s) during the inspection of your facility. They are inspectional observations and do not represent a final Agency determination regarding your compliance. If you have an objection regarding an observation, or have implemented, or plan to implement, corrective actions in response to an observation, you may discuss the objection or action with FDA representative(s) during the inspection or submit this information to FDA at the address above. If you have any questions, please contact FDA at the phone number and address above.
For all medical device inspections the FDA 483 should contain the following additional statement:
The observations noted in this form FDA 483 are not an exhaustive listing of objectionable conditions. Under the law, your firm is responsible for conducting internal self audits to identify and correct any and all violations of the quality system requirements.
b. Satellite Program Areas
Some program areas are considered satellites to the four major quality management system subsystems (Management Controls, Design Controls, CAPA, and P&PC):
CAPA Satellites:
- MDR
- Corrections & Removals
- Tracking
Production & Process Control Satellite
- Sterilization
Refer to the QSIT Guide for details on how to inspect those areas mentioned above. Refer to Part V of this Compliance Program for guidance on Regulatory and Administrative follow-up to these programs. Report the time spent on the Satellites under the appropriate corresponding PAC. Time for coverage of these satellites is averaged into the Level 1 and Level 2 inspectional work plan modules.
The following guidance should be used for determining when to cover the various programs.
QS should be covered duringeach inspection. Coverage is determined by the "level" of desired inspection. See Part III, above for guidance on which level to use and which subsystems to inspect.
MDR compliance should be covered during each inspection. Prior to initiating an inspection, the MDR data should be reviewed using eCIRS or go through CDRH to obtain information regarding the firm’s current reports. Be alert to the fact that MDRs may contain information on recalls that have not been reported through the district under 21 CFR Part 806.
Corrections & Removals . Determine during all QS inspections whether the firm has initiated any corrections or removals since the previous inspection and inspect for compliance with the Corrections & Removals regulation as described in the QSIT Guide. A Corrections & Removals inspection should also be initiated when a manufacturer is reporting corrections or removals in MDR reports or Part 806 reports. Be alert to the fact that MDRs may contain information on recalls that have not been reported through the district under 21 CFR Part 806.
Tracking.A tracking inspection is recommended, for devices that were issued a tracking order, each time CAPA is covered. To obtain Tracking information, refer to “Medical Device Tracking Guidance for Industry and FDA Staff” or access http://www.fda.gov/cdrh/comp/guidance/169.html.
Sterilization. When the P & PC subsystem is being inspected, sterilization should be chosen if not covered during the previous inspection unless:
- CAPA indicators of existing or potential problems are found with any other specific process; or,
- Other higher risk processes exist.
c. Sampling Records
The QSIT Guide includes instructions for sampling records for review. Sampling is an important tool for reducing the time spent reviewing records while being able to make statistically based inferences about the significance of the findings. The QSIT sampling table should be used for sampling records for evaluating the firm’s adherence to requirements and their procedures, not for performing data verification or analysis.
During Level 1 and 2 inspections, the review of the records may be terminated if objectionable conditions are observed before the entire sample is reviewed. A FDA 483 observation may be made that the objectionable condition was found and move on to the next part of the inspection. However, QSIT Guide instructions caution that not reviewing the entire sample may result in the loss of additional information which may be useful in understanding the potential prevalence of the objectionable condition, or the failure to identify other objectionable conditions.
During Level 3 inspections, however, the investigator and the compliance officer should work together closely to plan how sampling will be conducted. It is important for the compliance officer to be confident that the level of sampling will be sufficient to document the deficiency and support a potential regulatory action. During Level 3 inspections, it is recommended that the investigator review the entire sample of records to provide a complete picture of any deficiencies identified during sampling.
When evidence is collected utilizing the sampling tables, the EIR should reflect the following information:
- The type of records reviewed
- The sampling table used, Table 1 or 2
- The row used, row A, B, C, D, E or F
- The size of the sample and the number of records it was based on
- The number of records actually reviewed (may be the same as or different from the size of the sample)
- The results of sample review
Computer aided techniques may also be useful tools to efficiently evaluate electronic records (e.g. a large volume of complaint files) or accomplish assignment specific objectives (e.g. evaluating for trends in product specific complaint or failure data).
Note: Statistical support is available from CDRH, Office of Surveillance and Biometrics. DFI experts are available to assist with support in applying computer aided techniques.
3. Special Instructions Concerning Design Controls
The inspectional authority for review of design control records is derived from Section 704(e) of the Act. Such authority applies only after the establishment has manufactured the device for which the design has been under development or taken an action that precludes the argument that the product under development is not a device. Such action includes: (1) submitting to an Institutional Review Board plans for clinical investigation of the device; (2) submitting to FDA a Product Development Protocol (PDP); (3) submitting to FDA an IDE, 510(k), PMA, Humanitarian Device Exemption (HDE) or Premarket Report (PMR); and (4) changes to an already marketed device. Therefore, FDA has inspectional authority to review design control records when the device has been placed on the market or when any of the four actions above have occurred.
The above limitation does not apply to inspectional authority to review all generic design control procedures at any point in time.
Review of design controls should cover any design processes performed after June 1, 1997. The manufacturer is not required to retrospectively apply design controls to any stages in the design process that it had completed prior to June 1, 1997, unless changes have been made to the design (including changes in ownership or where the designed device will be manufactured) after June 1, 1997.
If a manufacturer normally designs its own devices, but has not initiated any design changes to current devices since June 1, 1997, or does not have a design project underway that is reviewable by FDA given the limitation discussed above, investigators should limit their coverage to a review of the design change control procedures that the manufacturer must have defined and documented.
There are a number of multi-establishment firms that conduct all design activities at a single facility (sometimes referred to as a research and development (R&D) center or corporate design facility). If the establishment scheduled for inspection is serviced by an R&D center or corporate facility, review the establishment jacket, before beginning the inspection, consult the agency’s on-line OEI databases and/or directly contact the district involved. Determine if the home district of the R&D center or corporate design facility has conducted a design control inspection of that facility within the previous two years. If such an inspection was conducted, it will not be necessary to conduct a design control assessment at the establishment scheduled for inspection. If an inspection was not conducted within the previous two years, issue an assignment to the home district of the R&D center or corporate design facility requesting a design control inspection.
Some manufacturers have their devices designed under contract. These manufacturers must comply with the requirements for using contractors or service suppliers under 21 CFR § 820.50 as well as ensuring compliance with 21 CFR § 820.30. The manufacturer must maintain or have reasonable accessibility to copies of a Design History File for any device that is in production.
Observations relating to Design Controls placed on the FDA 483 should be limited to the adequacy of and adherence to the procedures and/or controls established by the firm. Do not place observations on the FDA 483 that concern the adequacy, safety, or efficacy of a particular design. Any such concerns should be noted in the EIR and the EIR flagged for review by the Office of Device Evaluation or the Office of In Vitro Diagnostic Devices/CDRH.
4. Special Instructions for Sterilization Processes
Sterilization Process Controls section found in the QSIT Guide is a sub-part of the Production and Process Controls subsystem. The instructions for inspecting sterilization processes are applicable at the following types of facilities:
- device manufacturers that sterilize their own product
- device manufacturers that use contract sterilizers
- contract sterilizers
NOTE: The portion of the inspection spent covering sterilization processes should be reported under PAC 82845S.
Refer to Part III, A. 6, for guidance on collection of samples relating to sterilization issues.
5. Inspection of Radiation Emitting Devices
Medical Devices which are also deemed to be “electronic products” as defined by the Federal Food Drug and Cosmetic Act, Subchapter C – Electronic Product Radiation Control, section 531(2), may be inspected under this compliance program. These devices have additional Radiological Health requirements to protect the public from unnecessary radiation. The requirements include the affixing of certification labeling, additional reporting and record keeping, and the continued testing to verify product conformance with applicable Federal Performance Standards promulgated under 21 CFR 1020 - 1050. If the device being inspected is subject to Radiological Health requirements, follow the appropriate Compliance Program. Report any Radiological Health time under the appropriate Radiological Health PAC.
When conducting QS inspections, a firm may manufacture medical devices which are capable of emitting electronic product radiation. Based on district concurrence, the firm’s devices should also be assessed against the applicable standards promulgated under Chapter V, Subchapter C - Electronic Product Radiation Control of the FD&C Act. This assessment is not a QS activity and should not be reported as a QS activity.
Use Compliance Programs 7386.001, 7386.002; and 7386.004 through 7386.007 for guidance on inspections in this area. For Field Compliance Testing of Diagnostic Medical X-Ray Equipment, use CP 7386.003.
Device manufacturers subject to existing FDA performance standards (21 CFR Parts 1020 – 1050) should include in their device master and history records those procedures and records demonstrating compliance with the applicable standard, self-certification (21 CFR 1010), and reporting (21CFR 1002 – 1005).
6. Sample Collection
For QS, MDR, Tracking, and Correction and Removals violations , samples are not generally necessary to support a Warning Letter. However, the District office may require at least a documentary sample to support even a Warning Letter. Follow the district requirements. Also refer to IOM Section 5.6.1.2.
Samples may be required to support further action beyond a Warning Letter. The investigator should work with District management and compliance branch on deciding to collect samples to support QS violations. Physical samples should not be routinely collected to support QS cases. If the district should reference violative documentary or physical samples as evidence to support QS deviations, the sample should be tied to the QS deviation to show a cause/effect relationship.
Normally, the collection of samples for sterility issues is not to be performed during Level 1 (Abbreviated) inspections of device manufacturers or contract sterilizers. If sterility issues are in regards to packaging or seam integrity, sample collection may be needed. The following items provide guidance on sampling decisions. For questions regarding sterilization issues or the need to collect samples related to the sterilization process contact CDRH, Office of Compliance, at (240) 276-0115. Guidance on sampling decisions can be found in Part IV C.
- Finished device samples should not routinely be collected and tested for sterility to prove quality system deficiencies in sterilization validation or process control. Under certain circumstances, the Center may request that samples be collected for sterility testing.
- Field examination of packaging used for sterile devices may be indicated when the assessment of packaging operations demonstrates a lack of control such that inadequate packaging is likely to occur. Examine the packages for integrity of the sterility barrier, paying close attention to seals.
- Samples of defective packaging found during a visual field examination, if regulatory action is contemplated for packaging deficiencies, consist of 20 sterilized packaged devices.
- Bioburden samples are to be collected only 1) when the review of the results of bioburden testing performed by the manufacturer finds unrealistically low results; and, 2) the sterilization process is a bioburden based cycle with no safety overkill element. The sample is to consist of 20 unsterilized devices.
- Biological indicators are not to be collected routinely. Collect 40 biological indicators only if there is reason to question the effectiveness of the indicators or under direction by the Center.
- Endotoxin samples are to be collected only when endotoxin control is necessary for the device and when the review of the manufacturer's test methodology suggests that the manufacturer's test results may be unrealistically low. Collect 10 sterilized devices.
If the investigator is uncertain as to whether a sample should be collected, he/she should consult with district management who may consult with the CDRH Headquarters Laboratory Liaison, WEAC, or the Division of Field Science in ORA on the laboratory capability to conduct the analysis. (See Part VI, B. for program contacts).
1. Registration and Listing
Registration and Listing should be reviewed as part of the pre-inspectional activities and evaluated during inspections. Inspections should be limited to the minimum time and effort it takes to make an assessment. Review of a random sample of device listings (less than six) and the most recent registration is adequate. Also, randomly select two products from the firm’s catalog (or equivalent document) and determine whether listing was done. Assess whether these documents are up to date and correct.
NOTE: Registration and Listing should be covered during both domestic and foreign inspections. Per IOM section 5.2.3.3 do not place the violative findings for registration and listing on the FDA 483, but make verbal statements to the top management about the concerns at the close-out discussion. See Part V, Section E for regulatory considerations.
For specific guidance concerning device registration and listing requirements see IOM Subchapter 2.9 – Regulatory Submissions, section 2.9.2.1 Device Registration and Listing. See Exhibit 5-12 for a Summary Registration and Listing requirements for medical devices.
2. Imports
No import field examinations or sample collections are scheduled under this program.
3. Exports
The FDA Export Reform and Enhancement Act of 1996 amended Section 802 of the FD&C Act to allow an establishment to export unapproved Class III devices or Class II devices not cleared and subject to mandatory standards under Section 514, to any of those countries listed in Section 802 of the Act that authorize marketing, and to any other country if the device complies with the laws of that country without first obtaining FDA authorization. Section 802 also requires that any such device must be manufactured in "substantial conformity with current good manufacturing practice requirements.”
Section 801(e)(1) of the Act permits the importation of adulterated or misbranded devices, components, or accessories for further processing or incorporation into a finished device, provided that the device is subsequently exported and not sold or offered for sale in domestic commerce.
Chapter 9 of the Regulatory Procedures Manual and IOM Section 6.1.2 provide guidance on “import for export”, including record keeping requirements and the types of operations that qualify as further processing or incorporation of a component into a finished device. Exports under section 802 are subject to cGMP requirements found in the QS regulation.
Manufacturers are encouraged to make prior arrangements with their FDA district office before initiating an import for export operation. The review of the factory jacket should reveal when firms are performing such operations. The inspection should confirm that the firm is complying with the applicable requirements of the QS regulation for exports under section 802.
4. Electronic Records and Electronic Signatures
Follow agency policy when inspecting electronic records and signatures, see Part VI.
1. Remanufacturers of Used Devices
Remanufacturers are persons who process, condition, renovate, repackage, restore or do any other act to a finished device that significantly changes the finished device’s performance or safety specifications or intended use [21 CFR 820.3(w)]. Remanufacturers are considered to be manufacturers, and are subject to all applicable requirements of the Quality System regulation, MDR requirements, Device Tracking requirements, Registration and Listing, and premarket approval or clearance requirements. If an establishment disputes its regulatory status, the district should refer the EIR to the appropriate Division of Enforcement within CDRH/OC for assistance in interpreting the definition of a remanufacturer.
NOTE: For a discussion of the above issues see Federal Register Notice: December 23, 1997 (Volume 62, Number 246), pages 67011 – 67013.
2. Third Party Refurbishers/Reconditioners/Servicers of Used Devices
Third party refurbishers, reconditioners, servicers and "as is" remarketers of used devices are currently not subject to the requirements of the Quality System regulation. If the district receives an assignment to inspect such an establishment, the district should contact the Office of Compliance, Office of the Director (HFZ-300) at 240-276-0100 to determine the current regulatory status of such establishments.
3. Reprocessors of Single Use Devices
Third party reprocessors of single use devices are considered to be manufacturers and are subject to those requirements of the Quality System regulation that apply to the operations they perform. See Enforcement Priorities for Single-Use Devices Reprocessed by Third Parties and Hospitals, August 14, 2000, for guidance on FDA’s enforcement strategy. http://www.fda.gov/cdrh/reuse
The district should contact CDRH, Office of Compliance, Office of the Director (HFZ-300) at (240) 276-0100 for guidance before conducting an inspection of an establishment believed to be a third party reprocessor of single use devices, when not part of the assignment.
4. Hospital Reprocessors
Hospital reprocessors are to be only inspected under CDRH assignment.
1. General Reporting requirements are listed on the cover page. Refer to the IOM for EIR formats. Always include device, device class, and subsystems covered in the EIR.
2. QS Observations--If there are observed violations of the QS requirements, place them on the Form FDA-483. The QSIT Guide provides guidance concerning major QS requirements and the identification of major deviations. The most serious system deficiencies should be noted on the Form FDA-483 first, then by subsystems if possible. Special Note: Refer to the IOM for information concerning annotation of the Form FDA-483.
3. 510(k) or PMA Observations--If the establishment does not have a valid:
- PMA for a device that is offered for introduction into interstate commerce;
- 510(k) for a device that was offered for introduction into interstate commerce for the first time after May 28, 1976; or,
- Has made significant changes to a device that require a new 510(k), or PMA supplement
then investigators should not place the observations on the Form FDA-483 unless concurrence is obtained from CDRH/OC and/or OIVD. When Center concurrence cannot be obtained before the inspection is completed, investigators are requested to obtain complete documentation and submit that documentation for CDRH review through the district compliance branch.
4. Registration and Listing Observations -- If a firm has failed to list device(s), or to verify that their listings are up-to-date every six months and update them if they are not, as required by 21 CFR Part 807, make note of this observation(s) in the EIR for consideration for action by the district Compliance Officer. If a firm has failed to renew its annual registration for the last two or more years as required by 21 CFR Part 807, make note of this observation in the EIR for consideration for action by the district Compliance Officer. All registration and listing observations should be reported to firm management.
NOTE: A firm's registration and listing status can be determined by querying the CDRH Registration and Listing database through OSCAR or eCIRS.
Field Accomplishments and Compliance Tracking System (FACTS)--Refer to existing policy in the IOM.
5. FDA Field Accomplishments and Compliance Tracking System (FACTS)a. When selecting specific manufacturing processes to represent profile classes, investigators should give preference to:
- CAPA indicators of process problems
- Process used to manufacture high risk products
- Processes that have a high risk of causing product failure
- Processes that require process validation
- Processes that are new to the manufacturer
- Processes that cover a variety of process technologies and profile classes
- Common process used in multiple products
- Processes not covered during previous inspections
NOTE: If all profile classes are not directly covered during an inspection, but are covered indirectly under CAPA, then all profile classes the firm is involved with can be listed on the appropriate FACTS screen.
b. Quality System Inspections conducted should include:
(1) coverage of the device(s) specified in the assignment, or devices and related manufacturing processes representing all the same profile classes as the assigned device; and,
(2) other devices as required to provide coverage of any remaining profile classes, except QS exempt Class I devices.
c. Since the QSIT approach covers systems, the findings from the inspection can apply to all profile classes at the firm.
The district will make all the necessary arrangements for proper handling of samples with the following designated testing facilities:
| TYPES OF DEVICES | ANALYZING LABORATORIES |
|---|---|
| All General Medical Devices | Winchester Engineering and Analytical Center (WEAC) 109 Holton Street Winchester, Massachusetts 01890-1197 |
| Radioimmunoassay | WEAC |
| All Other In Vitro Diagnostic Devices | Micro - WEAC Chem - WEAC |
| Testing for sterility of finished devices,
package integrity, bioburden,
and endotoxins: |
WEAC |
| Testing of biological indicators: | WEAC |
See PART VI regarding those persons designated as contacts for WEAC and specific products.
SPECIAL NOTE: For all other devices and questions concerning sampling of devices and laboratory capabilities, contact Division of Field Science (DFS), HFC-140.
Sample collection and analysis will be determined on a case-by-case basis through consideration of inspectional findings, compliance and scientific capabilities and expertise. Full collaboration between investigations and analytical personnel is essential. See Part III for additional information.
1. Testing Finished Device Samples for Sterility
a. Visually examine each unit to ascertain that its packaging is intact. Report all defects observed by describing the size, type and location of the defects. Units with defective packaging need not be examined for sterility.
b. Finished device samples are to be tested in accordance with the requirements of current USP methodology for Sterility Tests. Reference the FDA Sterility Analytical Manual for guidance on applying the USP methods.
c. Device samples are to consist of 60 units, as follows:
20 units tested in Soybean-Casein Digest Broth
20 units tested in Fluid Thioglycollate Broth
10 units for bacteriostasis/fungistasis testing
2 units for system control
8 units for method development
60 units for re-test, if required under USP methodologyWhen 120 units are not available because of lot size or cost, follow the current USP recommendations for the minimum number of articles to be tested in each media, as follows:
Number of Articles
in the BatchNumber of Articles
to be testedNot more than 100 articles 10% or 4 articles,
whichever is greaterMore than 100, but not more
500 articles10 articles More than 500 articles 2% or 20 articles
whichever is lessNote that the USP permits the division of articles into equal portions for addition to each of the specified media when the contents of the article are of sufficient quantity (see the current USP to determine what is a sufficient quantity).
NOTE: For the purposes of this compliance program, the “articles” referred to in the USP may be interpreted as devices.
d. Positive subsamples
Check cultures for growth daily and begin qualitative analysis of growth immediately upon detection of growth. Follow subculturing procedures in the Sterility Analytical Manual. Continue to incubate growth vessels after subculture for full term analysis to detect slow growing bacteria and molds. For each subsample found to be non-sterile, prepare a pure culture of each contaminant. All isolates from sterility tests must be maintained until otherwise notified by CDRH or for one year.
2. Presterilization Microbial Contamination (Bioburden)
Bioburden testing is to be performed in accordance with the guidance provided in ISO 11737-1, Sterilization of medical devices - Microbiological methods - Part I: Estimation of population of microorganisms on products. The methodology used for estimating the bioburden is to be validated. Twenty units are to be tested.
3. Analysis of Biological Indicators
Test 40 biological indicators according to current USP methodology using sterilization conditions specified on the indicator label. "Survival time and kill time" and "Resistance performance tests" are to be used. 80 additional biological indicators may be required if either performance test fails. Under some conditions, the D-Value may also be determined. That determination requires a minimum of 45 biological indicators. These determinations will be performed according to the claims of the manufacturer of the indicator or inoculated product. Pertinent test specifics will be required.
4. Analysis of Packaging Defects
Perform a visual, non-destructive, inspection of the package noting the existence and location of seal or material defects. Normally 20 packaged devices will be collected for analysis. Further testing is to be performed using consensus standards such as those identified in the Part VI.A.1 references for the American Society for Testing and Materials (ASTM). Selection of the test will depend on the materials and construction of the package, and on the nature of the noted or suspected problem.
5. Analysis of Endotoxins
Samples will be analyzed using the Bacterial Endotoxins Test found in the current USP and the Sterility Analytical Manual. Ten units are required for endotoxin testing.
6. Antimicrobial Effectiveness Testing
Samples will be analyzed using the Antimicrobial Effectiveness Test found in the current USP and the Sterility Analytical Manual. Ten units are required for testing.
1. Compliance Decision
a. Situation I
The district has documented evidence indicating that one or more major deficiency with the Quality System regulation has resulted in the inspection being classified as Official Action Indicated (OAI). Examples that may be considered include:
- Total failure to define, document, or implement a quality system or one of the seven subsystems. The following list only provides examples and is not all-inclusive:
- No procedure(s) which address corrective and preventive actions.
- No procedure(s) on how all the quality data will be analyzed and utilized.
- Where design controls are required, no design control procedure(s) for a particular device or family of devices, i.e., only high level design control procedures.
- Where design controls are required, no design change control procedure(s).
- No documented process validation for a process(s) the results of which cannot be fully verified.
- A deficiency in one or more element(s) of the subsystems. The QSIT Guide focuses on the most important aspects within each subsystem and can be utilized to determine what the Agency believes is critical and therefore would constitute “major” problems if not adequately addressed. Particular attention should be paid to the relationships of requirements. For example, deficiencies in both purchasing controls and acceptance activities can indicate a major deficiency because control of components and suppliers depends on a mix of both of these activities and if there are problems with one or both, assurances are greatly diminished.
- The existence of products which clearly do not comply with the manufacturer’s specifications and/or the Quality System regulation and which were not adequately addressed by the Corrective and Preventive Actions Subsystem (CAPA) program.
- Noncorrection or inadequate correction of major deficiencies from previous inspection(s). Repeat deficiencies of same or similar deficiencies from previous inspection(s).
If any major deficiencies exist, the district is expected to classify the EIR as OAI and, based on the significance (risk) of the device and the findings, the district should consider which administrative and/or regulatory action to initiate. Such actions include, but are not limited to, issuance of a Warning Letter, injunction, detention, seizure, civil penalty and/or prosecution. See Regulatory Procedures Manual for further guidance.
If any of these deficiencies exist for foreign manufacturers, based on the significance (risk) of the device and the findings, a Warning Letter and/or Warning Letter with Detention without Physical Examination will be considered by CDRH/OC.
IMPORTANT NOTE: If a serious health hazard is identified, and the firm is not cooperative in conducting a voluntary recall, an FDA mandated recall (Section 518(e) of the FD&C Act), administrative detention/seizure or injunction should be considered as the initial action to bring the situation under prompt control.
b. Situation II
The inspection documents QS deficiencies of a quantity and/or type to conclude that there is minimal probability -- in light of the relationship between quality system deficiencies observed and the particular device and manufacturing processes involved -- that the establishment will produce nonconforming and/or defective finished devices. The Form FDA-483, Inspectional Observations, will serve to inform the establishment of any objectionable findings.
IMPORTANT NOTE: A Situation II should not be assigned if the inspection documented major deficiencies and the firm responds only with promised corrections, corrective actions and preventive actions. In order for an inspection to be classified as Situation II, FDA must have documented evidence of effectively implemented corrections and corrective actions taken on any and all major deficiencies observed during the inspection.
2. Contract Sterilizers, Contract Device Manufacturers and Finished Device Manufacturers – Deciding Responsibility When Taking Regulatory Action
a. The following is provided as guidance for deciding which party is to be held responsible when a finished device manufacturer uses a contract sterilizer to perform terminal sterilization on its devices or a contract device manufacturer:
- Contract sterilization and contract manufacturing are considered an extension of the finished device manufacturer's process. The finished device manufacturer is ultimately responsible for assuring that validations, operations, process controls, quality assurance checks, etc. are appropriate, adequately documented and correctly performed.
- Contract sterilizers and contract manufacturers of finished devices are considered manufacturers for the purpose of applying the Quality System regulation in that they meet the definitions as described in 21 CFR § 820.3(l) finished device and 21 CFR § 820.3(o) manufacturer. Contract sterilizers and contract manufacturers of finished devices are subject to those parts of the Quality System regulation that apply to the operations that are performed.
- The finished device manufacturer bears overall responsibility for the safety and effectiveness of the finished device and must control all contractors under 21 CFR § 820.50 Purchasing controls and 21 CFR § 820.80 Receiving, in-process, and finished device acceptance. However, a contract sterilizer/contract manufacturer of finished devices and the finished device manufacturer are all legally responsible for compliance with the Quality System regulation and for assuring the safety and effectiveness of the finished device.
- Contract manufacturers, to include contract testing or contract laboratories, that are not manufacturing a device meeting the definition of a finished device in 21 CFR § 820.3(l) are not required to meet the Quality System regulation. These contractors, even though they may meet the definition of a “manufacturer,” are to be controlled by the finished device manufacturer under 21 CFR § 820.50 Purchasing controls and 21 CFR § 820.80 Receiving, in-process, and finished device acceptance.
- For contract sterilization, the written agreement, between the manufacturer and contract sterilizer, required by 21 CFR 801.150(e), may be referenced to determine how the parties have defined their respective responsibilities. For other contract manufacturers, any written agreements used as part of supplier controls under 21 CFR § 820.50, may be referenced to determine how the parties have defined their activities and respective responsibilities.
b. When deviations are observed, proposed regulatory actions should reflect and identify the shared responsibilities between the contractor and finished device manufacturer. In some situations, it may be appropriate to initiate regulatory action against both the contractor and the device manufacturers:
- Appropriate action should be considered against the contract sterilizer or contract manufacturer of finished devices in areas for which it has the prime responsibility under any written agreement. It may be necessary to inspect more than one customer to develop supporting documentation to demonstrate the particular contractor does not appear to have adequate controls.
- When an inspection of a contractor finds violations in areas that are the responsibility of the finished device manufacturer (such as validation, biological indicators, package seal testing, etc.), these deviations are to be reported to the home district of the finished device manufacturer. Regulatory action consistent with the action of choice for the contractor should be considered for the finished device manufacturer.
- Because the finished device manufacturer is ultimately responsible for the safety and effectiveness of the device and therefore the contractor's activities, serious deficiencies found at a contractor’s establishment will indicate consideration of regulatory action against the finished device manufacturer. Copies of Warning Letters issued to a contract sterilizer or contract manufacturer of finished devices should be sent to the finished device manufacturer with appropriate redaction. A copy should also be sent to the home FDA district office of the finished device manufacturer. These documents should be used as a basis for the next scheduled inspection of the finished device manufacturer.
- When a possible health hazard situation exists due to the contractors operation; or an administrative or legal action is contemplated against a contract sterilizer or contract manufacturer of finished devices, the home FDA district office(s) of all finished device manufacturers utilizing that contractor should schedule an immediate follow-up inspection at all affected device manufacturers.
3. Violative Devices Sold to Government Agencies
It is agency policy to treat devices sold to the federal government in the same manner as devices sold to commercial accounts. Consequently, when FDA recommends against acceptance of a device by a government agency because that device, or its manufacturer, is in violation of the FD&C Act, FDA should also recommend appropriate regulatory/administrative action against the same or similar device sold to commercial accounts.
If an establishment has shipped a violative device to a Government agency, appropriate regulatory action consistent with the nature of the violation(s) may be taken even though there have been no shipments to commercial customers. Formal regulatory action in connection with a violative shipment may not be necessary in some cases. (For example, the establishment promptly corrects the violative condition, and the Agency would not require further action if the matter involved a device shipped to a non-government customer). However, where corrections are not or cannot be made promptly, the main concern is preventing the subsequent shipment of the device to another customer. When the device has been shipped solely to a Government agency and is under control of that agency and there is no threat to the public, the ORA/Division of Compliance Information and Quality Assurance (DCIQA) staff should ascertain the intention of the agency holding the goods (e.g., will they return or destroy the goods; will they request FDA to initiate seizure, etc.). If the procuring agency requests FDA action, ORA DCIQA staff will refer the matter to the home FDA district office for their consideration of an appropriate recommendation.
4. Administrative and Judicial Actions
Actions which may be considered include: FDA requested recall, FDA mandated recall, Warning Letter, seizure, injunction, prosecution, civil penalties and detention.
Corrections and corrective action proposals and documented evidence of those corrections and corrective actions should be submitted by a responsible official of the establishment in writing, detailing the action(s) taken and to be taken to bring the violative process or product into compliance within a specified time frame. Voluntary correction does not preclude the initiation of administrative and/or judicial action.
In determining whether quality systems deviations are sufficient to support legal action, consideration should be given to the significance of the device, the establishment's quality history, and whether the problem(s) is widespread or continuing.
a. Warning Letters
Issuance of all Warning Letters should follow Chapter 4 of the Regulatory Procedures Manual (RPM) http://www.fda.gov/ora/compliance_ref/rpm/. Consult the Office of Enforcement’s (OE) Warning Letter page on ORA’s intranet website for current instructions for obtaining Office of Chief Counsel (OCC) clearance and for current approved Warning Letter templates.
Districts have DIRECT REFERENCE AUTHORITY for Warning Letters in certain areas which are described in Chapter 4 of the RPM.
NOTE: Regarding direct reference authority for Correction and Removal violations, Warning Letters should only be issued once the districts have checked with their District Recall Coordinator to confirm that the recall is Class I or II.
Districts should obtain CDRH concurrence before issuing Warning Letters related to refurbishing/reconditioning of used devices, reprocessing of single use devices, violations of Part 11 relating to of Electronic Records and Electronic Signatures and other areas as prescribed in Chapter 4 of the RPM.
If the district determines that issuance of the Warning Letter has resulted in appropriate corrections and corrective action by the establishment, the district should, within five (5) working days after confirmation of documented evidence, update the establishment's profile data in FACTS.
b. Violative Follow-Up Inspections
As stated in Part III of this Compliance Program, the post-inspection activities serve to advise manufacturers that the conditions identified by the investigator may be symptomatic of system problems, and that the manufacturer is responsible for investigating, identifying, and correcting system problems. The Warning Letter templates further direct the establishment to discuss in its response how it will address the system problems related to the conditions identified by the investigator.
After issuance of a Warning Letter for Quality System violations, the next inspection should be a Level 3 inspection, as explained in Part III of this program and coverage is dependent upon whether the previous inspection was Level 1 or Level 2 as explained in that Part. When investigators identify the same or additional conditions that meet the criteria for Situation I, the district should consider subsequent enforcement actions, such as seizure, injunction, prosecution, or civil penalties. During Level 3 inspections, the investigator should work closely with the district compliance officer and where appropriate CDRH to assure that appropriate coverage is provided and deviations properly documented.
c. The Recidivist Policy -- Enforcement Strategy For Establishments With Repeated Violative Inspections
(1) Some establishments have a high rate of recidivism. They have developed a pattern of correcting violative conditions in response to a Warning Letter or other administrative/regulatory action, and usually maintain those corrections long enough to pass the follow-up inspection. When FDA next inspects the establishment (sometimes, as a follow-up to a recall), the investigator identifies similar conditions that again meet the criteria for Situation I. This tendency toward recidivism is often due to the failure of the establishment to have an effectively established quality management system being implemented.
(2) When dealing with another violative inspection for such an establishment, the district should consider using the following strategy:
(a) Issue a Warning Letter that follows the Recidivist Warning Letter approved template found on OE’s Warning Letter page on the ORA intranet website. This Recidivist Warning Letter requests the manufacturer to submit to the district (for up to 2 years if the district believes that it is necessary) an annual certification by an outside expert consultant stating that it has conducted a complete audit of the establishment's quality management system relative to the requirements of the Quality System regulation. The manufacturer should submit a copy of the consultant's report1, and certification by the establishment's CEO that he or she personally has received and reviewed the consultant's report and that the establishment has made or taken all corrections and corrective actions identified in the report. To keep the process on track, schedules, milestones, update reports and other similar activities should be established between the firm and FDA, or by the firm after issuance of the Recidivist Warning Letter.
(b) Compliance Officers have the option of limiting the review of the certification only to the extent necessary to confirm that the consultant and the establishment have met the requirements set forth in the Recidivist Warning Letter. Compliance Officers may also request a technical evaluation of the consultant's report by the appropriate branch within the Office of Compliance (OC) or Office of In Vitro Diagnostics (OIVD) at CDRH. Compliance Officers have no obligations, however, to send to the establishment comments regarding the adequacy of the consultant's report or the establishment's corrections.
(c) Follow-up inspections will normally be conducted 3 – 6 months after the establishment certifies that it has completed all corrections and corrective actions.
(d) If the follow-up inspection indicates that the corrections and corrective actions are satisfactory, the district should notify the establishment that it has no objections. The district office should update the profile data. The district should also remind the establishment that it should continue to submit to the district, in accordance with the schedule specified in the Recidivist Warning Letter, certification by an outside expert consultant that it has conducted an updated audit, has certification by the establishment's CEO that any corrections and corrective actions noted to be necessary by the consultant have been made, and remains in compliance with the requirements of the Quality System regulation. The establishment should continue to submit copies of the audit results.
(3) If conditions identified by the immediate follow-up inspection or subsequent inspections meet the criteria for Situation I, the district should consider action such as injunction or seizure per A.1 above and the RPM.
(4) If the evidence indicates that the consultant's or establishment's certifications are fraudulent, the district is encouraged to advise and seek assistance from the Office of Criminal Investigations. When there is clear evidence that the establishment falsified its status report to the district, the district should initiate appropriate action under 18 USC 1001.
d. Recalls
If the district believes that prompt removal of a violative device from channels of commerce is necessary, it should proceed in accordance with the requirements of 21 CFR § 806 and established recall procedures found in Chapter 7 of the RPM and 21 CFR Part 7 (Enforcement Policy), Subpart C (Recalls). In the event of serious adverse health consequences or a death, CDRH may order a firm to discontinue further distribution and advise customers of the problem, and may subsequently order the recall of a device to the user level in accordance with Section 518(e) of the Act.
e. Seizure
A seizure is an action that is intended to take quick control over the violative product and put it under the possession or custody of the Court. A seizure should be recommended if appropriate, as stated in Chapter 6 of the RPM.
f. Administrative Detention/Seizure
Prior to invoking an administrative detention, for a period of 20 or 30 days, the district director should have reason to believe: (1) the device is misbranded or adulterated; (2) the establishment holding the device is likely to quickly distribute or otherwise dispose of the device; and (3) detention is necessary to prevent use of the device by the public until appropriate regulatory action may be taken by the Agency.
District Directors should consult via telephone with CDRH, OC, Office of the Director and the Office of Chief Counsel (OCC) concerning administrative detention. Concurrence should be given by the Director, OC, CDRH, based on a recommendation by the OC and/or OIVD staff and OCC staff.
The district should immediately recommend seizure of the detained devices to assure continued control of the violative device after the 20/30 days of administrative detention expire.
g. Injunction
If an establishment has a continuing pattern of significant deviations in spite of past warnings, injunction will usually be the recommended action of choice. If a serious health hazard exists, the recommendation should include a request for a temporary restraining order (TRO) to prevent the distribution of devices that have been manufactured under the violative conditions documented by the inspection report per the instructions in Chapter 6 of the RPM.
The recommendation should be accompanied by copies of all necessary documents, e.g., complete inspection reports, Warning Letters issued, sample analyses reports, establishment's response(s) to Warning Letters and/or Form FDA-483.
In the absence of physical samples, the inspectional evidence should clearly show that the establishment has deviated from the requirements of the Quality System regulation and/or other regulations and the establishment meets the requirements of OAI. These deviations should be well documented and should show continuing system deficiencies, not just an isolated event.
h. Citation
A citation should be recommended, if appropriate, as stated in Chapter 5 of the RPM.
i. Prosecution
The criteria stated in Chapter 6 of the RPM are the criteria for consideration of prosecution of individuals in violation of the requirements of the Quality System regulation.
j. PMA Disapproval/Withdrawal
Refer to Compliance Program 7383.001, Part V.
k. Detention without Physical Examination
In general, detention without physical examination should be recommended by the Office of Compliance whenever there is documented evidence of an OAI situation for a foreign manufacturer when the criteria for a domestic seizure, injunction, or other regulatory remedies beyond a Warning Letter are met.
l. Civil Money Penalties
Section 303(f)(1)(B)(i) of the Act states that civil money penalties shall not apply to QS violations “unless such violation constitutes (I) a significant or knowing departure from such requirements, or (II) a risk to public health.” Section 303(f)(1)(B)(iii) further stipulates that civil penalties shall not apply to “section 501(a)(2)(A) which involve one or more devices which are not defective.”
For additional information, see the draft “Guidance for FDA Staff: Civil Money Penalty Policy” at http://www.fda.gov/cdrh/comp/penalty.pdf. Also refer to “Guidance for Industry and FDA Staff: Reduction of Civil Money Penalties for Small Entities” at http://www.fda.gov/OHRMS/DOCKETS/98fr/010049gd.pdf.
5. Facilitating Review of Regulatory Recommendations
a. The district should contact the appropriate CDRH/OC Division Director or the CDRH/OIVD Deputy Director by phone when the district believes they have an OAI situation for which a recommendation for seizure, injunction, civil penalties, or prosecution may be appropriate.
CDRH fully supports the concept of “Up Front” loading so as to be fully aware of a potential situation and to provide guidance on how to proceed. At the discretion of the district, notification to CDRH may occur prior to an inspection, while the inspection is ongoing, or after issuance of the Form FDA-483. Notification would typically be made by a compliance officer, but could be made by the investigator and/or district management. The CDRH/OC and CDRH/OIVD organization charts are shown in Attachment A and B.
b. When the district knows a regulatory action will be recommended as a result of the inspection, it should FAX a copy of the issued Form FDA-483 to the appropriate division in OC or OIVD. The review process can begin within CDRH while the EIR and recommendation are being written by the district. A copy of the Form FDA-483 annotated with exhibit numbers, and EIR page numbers, helps the reviewers.
c. It is the responsibility of district management to ensure that the documentation and evidence presented with each legal action recommendation is sufficient to justify and support each charge. The material submitted should include only the basic documentation needed to support each QS charge/example.
d. All necessary samples and other supporting documentation should be tabbed and their location cross referenced in the recommendation in order to assist in a timely review. It is highly recommended that you provide a table that cross references the violation with the Form FDA-483 item number, the inspection report page number and the exhibit number.
e. All significant questions, problems, or other weaknesses in the evidence regarding the recommended action should be stated, along with pertinent district comments. Deficiencies/observations should be presented in descending order of importance.
f. The recommendation should begin with the most serious deviation from the regulations with reference to the EIR pages, exhibits and sample results that document the violation. Each charge should be parenthetically referenced in the recommendation memorandum and the page location of the supporting evidence given. Each deviation should be related to its effect on device quality in light of overall controls, and should be separated according to the type of manufacturing activity.
g. Physical samples are not required to support QS deviations, and should not be routinely collected for QS cases. If the district should reference violative documentary or physical samples as evidence to support QS deviations, the results should be tied to the QS deviation to show a cause/effect relationship.
h. Information regarding previous warning and other past or ongoing regulatory actions should be referenced along with a description of corrections and corrective actions. If the recommendation or current EIR references a previous report, the district should copy the cited EIR pages.
i. All legal action recommendations shall be sent to CDRH/HFZ-306 for processing.
The district should consider a Warning Letter when the following MDR violation(s) was disclosed during the inspection. This list only provides examples and is not all-inclusive.
When the firm has already received a Warning Letter for MDR violations and still fails to comply with the MDR regulation, then the district should consider recommending a seizure, injunction, civil money penalty or prosecution.
All failures to comply with MDR should be listed on the FDA-483.
IMPORTANT NOTE: Warning Letters based on failure to report malfunctions should have CDRH review/concurrence per the instructions in Chapter 4 of the RPM.
The district should consider a Warning Letter when the following tracking violation(s) was disclosed during the inspection. This list only provides examples and is not all-inclusive.
When the firm has already received a Warning Letter for tracking violations and still fails to comply with the tracking regulation, then the district should consider recommending a seizure, injunction, civil money penalty or prosecution.
All failures to comply with the tracking regulation should be listed on the FDA-483.
IMPORTANT NOTE: CDRH concurrence is required for a warning Letter for any violation of device tracking regulation requirements other than failure of the firm to implement any form of tracking system per the instructions in Chapter 4 of the RPM.
The district should consider a Warning Letter when the following Corrections and Removals regulation violation(s) was disclosed during the inspection. This is only an example and is not all-inclusive.
When the firm has already received a Warning Letter for Corrections and Removals violations and still fails to comply with the Corrections and Removals regulation, then the district should consider recommending a civil money penalty or prosecution.
All failures to comply with the Corrections and Removals regulation should be listed on the FDA-483, once the investigator has confirmed with their District Recall Coordinator that the situation would likely be classified as a Class I or II recall situation.
Chapter 4 of the RPM states agency policy is that Warning Letters should only issue for violations of regulatory significance. Generally, registration and listing violations, as a sole finding, should not be the basis of a warning letter.
However, when those violations are found in combination with other findings, such as quality system violations, they should be included on the Warning Letter, after CDRH concurrence.
Refer to Part V in Compliance Programs 7385.014, 7386.001, 7386.002; and 7386.004 through 7386.007 for guidance on regulatory actions related to radiation emitting devices.
When violations meet the criteria for Situation I for those unapproved devices exported under Section 802, note that fact in the Warning Letter. Submit a copy of the Warning Letter to CDRH, Division of Risk Management Operations, Regulatory Policy and Systems Branch (HFZ-307) with a recommendation to rescind all current or unexpired certificates of export.
Copies of CDRH QS publications and FDA guidance documents are available from the Division of Small Manufacturers International and Consumer Assistance (DSMICA), Telephone: 800-638-2041 or FAX 301-443-8818. Many of these publications are also available in the CDRH Good Guidance Practices (GGP) Database (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfggp/search.cfm).
Sources to obtain copies free of charge:
Internet (World Wide Web): FDA, CDRH, and ORA maintain web sites for easy access to information. The FDA home page is http://www.fda.gov; the CDRH home page is http://www.fda.gov/cdrh/; and the ORA home page is http://www.fda.gov/ora/.
Good Guidance Practices (GGP) Database: This is a searchable database that contains all current CDRH guidance documents and provides links to the documents.
(http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfggp/search.cfm)
The following sources may be referenced for further guidance regarding sterilization processes
Food and Drug Administration:
Guideline on Validation of the Limulus Amebocyte Lysate Test as an End-Product Endotoxin Test for Human and Animal Parenteral Drugs, Biological Products, and Medical Devices, December 1987 (http://www.fda.gov/cder/guidance/old005fn.pdf)
Updated 510(k) Sterility Review Guidance K90-1; Guidance for Industry and FDA, August 30, 2002 (http://www.fda.gov/cdrh/ode/guidance/361.pdf)
A searchable database of FDA-recognized standards is available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/search.cfm
A list of FDA-recognized standards related to sterilization of medical devices can be obtained by searching on the category “Sterility.”
United States Pharmacopeia (USP)/National Formulary (NF), current edition:
U. S. Pharmacopeial Convention, Inc.
12601 Twinbrook Parkway
Rockville, Maryland 20852
http://www.usp.org
http://www.uspnf.com (USP/NF Online)<61> Microbial Limit Tests
<71> Sterility Tests
<85> Bacterial Endotoxins Test (LAL)
<151> Pyrogen Test (USP Rabbit Test)
<161> Transfusion and Infusion Assemblies and Similar Medical Devices
<1211> Sterilization and Sterility Assurance of Compendial Articles
<1035> Biological Indicators for Sterilization
<55> Biological Indicator - Resistance Performance Tests
Biological Indicator for Dry-heat Sterilization, Paper Carrier
Biological Indicator for Ethylene Oxide Sterilization, Paper Carrier
Biological Indicator for Steam Sterilization, Paper Carrier
Biological Indicator for Steam Sterilization, Self-Contained
1. ORA Contacts
a. Questions regarding inspectional requirements and/or technical assistance:
Division of Field Investigations
Medical Device Group
Telephone: (301) 827-5645b. Questions about accessing or connecting to the CDRH Center Information Retrieval System (CIRS):
Employee Resource & Information Center (ERIC)
Telephone: (301) 827-ERIC (3742)
http://eric.fda.govThe current procedure for ORA is to request access to enhanced CIRS via ERIC. OITCDRH will 1) create an Oracle account, 2) enter user's name to a table that is used by the single sign-on, 3) install the Jinitiator. After these three things completed, user can access enhanced CIRS through the enhanced CIRS link in the CenterNet.
c. Questions regarding sampling of devices and laboratory capabilities:
William Campanaro or Lydia Rosas-Marty
Division of Field Science (DFS), HFC-140
Telephone: (301) 827-7605d. WEAC contacts for testing medical devices:
Laurence Coyne, Ph.D., Director
Engineering Branch, HFR NE480
Telephone: (781) 729-5700, ext. 761Pamela Mackill, Director
Analytical Branch, HFR NE460
Telephone: (781) 729-5700, ext. 748e. Questions regarding COMSTAT:
Gillie Kovalsky
Division of Compliance Information and Quality Assurance (DCIQA)
HFC-240
Telephone: (240) 632-68173. CDRH Contacts
NOTE: Refer to the CDRH/OC and OIVD Organizational Charts Attachment A and B respectively, to identify the unit within OC or OIVD that is responsible for the type of device for which you have a question or need guidance.
a. MDR Regulation Interpretation and Policy Questions:
Reporting Systems Monitoring Branch, HFZ-533
Division of Surveillance Systems, OSB
Telephone: (301) 594-2735Data retrieval of MDR reports:
Information Analysis Branch, HFZ-531
Division of Surveillance Systems, OSB
Telephone: (301) 827-2983b. Questions regarding sampling and/or testing of general medical devices:
Thomas R. Lee
Office of Science and Engineering Laboratories, HFZ 160
Telephone: (301) 827-4993
Email: thomas.lee@fda.hhs.govc. Express Mail Address for All Regulatory Action Recommendations:
Field Operations Branch, HFZ-306
Office of Compliance
Center for Devices and Radiological Health
2094 Gaither Road
Rockville, Maryland 20850d. Questions regarding the interpretation and applicability of the device Quality System regulation and GMP exemptions:
Kimberly A. Trautman
Quality Systems/GMP Expert, HFZ-340
Telephone: (240) 276-0296
Email: kimberly.trautman@fda.hhs.govJan Welch
Quality System/IVD Expert, HFZ-320
Telephone: (240) 276-0354
Email: jan.welch@fda.hhs.gove. Questions regarding remanufacturing, refurbishing/reconditioning of used devices:
Casper Uldriks
Office of Compliance, HFZ-300
Telephone: (240) 276-0106
Email: casper.uldriks@fda.hhs.govf. Questions regarding the reprocessing of single-use devices:
Larry D. Spears
Office of Compliance, HFZ-300
Telephone: (240) 276-0100
Email: larry.spears@fda.hhs.govg. Questions regarding this Compliance Program:
Kimberly A. Trautman
Quality Systems/GMP Expert, HFZ-340
Telephone: (240) 276-0296
Email: kimberly.trautman@fda.hhs.govh. Questions regarding compliance of product software, stand alone software, or process equipment software:
John F. Murray
Office of Compliance Software Expert, HFZ-340
Telephone: (240) 276-0284
Email: john.murray@fda.hhs.govi. Questions regarding sterilization should be directed to:
Patrick Weixel
Division of Enforcement A, HFZ-333
Telephone: (240) 276-0355
Email: patrick.weixel@fda.hhs.govCandace McManus
Division of Enforcement A
HFZ-333
Telephone: (240) 276-0345
Email: candace.mcmanus@fda.hhs.govj. Questions regarding Electronic Records and Electronic Signatures should be directed to:
John F. Murray
Division of Enforcement B, HFZ-340
Telephone: (240) 276-0284
Email: john.murray@fda.hhs.govk. Questions regarding potential or proposed regulatory actions should be directed to the appropriate CDRH/OC Case Expert:
Louis J. Kaufman
Division of Enforcement A, HFZ-320
Telephone: (240) 276-0151
Email: louis.kaufman@fda.hhs.govAndrea P. Latish
Division of Enforcement B, HFZ-340
Telephone: (240) 276-0294
Email: andrea.latish@fda.hhs.govl. Questions regarding compliance issues concerning in vitro diagnostic devices:
James Woods
Deputy Director, Patient Safety
Office of In Vitro Diagnostic Devices, HFZ-440
Telephone:240-276-0443 ext. 177
Email: james.woods@fda.hhs.gov4. FDA Web Sites :
a. FDA home page: http://www.fda.gov
b. ORA home page: http://www.fda.gov/ora/
c. CDRH home page: http://www.fda.gov/cdrh/
d. MDR: http://www.fda.gov/cdrh/mdr
e. MedWatch: http://www.fda.gov/medwatch
http://www.fda.gov/med watch/report/instruc.htm
(Instructions for completing MedWatch Form 3500A)f. QSIT Guide:
http://www.fda.gov/ora/inspect_ref/igs/qsit/qsitguide.htmg. FDA Recognized Standards: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/search.cfm
NOTE: A list of FDA-recognized standards related to sterilization of medical devices can be obtained by searching on the category “Sterility.”
h. The Biologics and Devices Intercenter Agreement:
http://www.fda.g ov/oc/ombudsman/bio-dev.htmi. Electronic Records and Electronic Signatures:
http://www.fda.gov /ora/compliance_ref/part11/j. Field Accomplishments and Compliance Tracking System (FACTS):
http://web.ora.fda.gov/factsite/default.htmk. Medical Device Tracking:
http://www.fda.gov/cdrh/comp/guidance/169.htmll. Registration and Listing Database (files to be downloaded):
http://www.fda.gov/cdrh/comp/estregls.htmlm. Establishment Registration Database (searchable):
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfrl/registration.cfmn. Device Listing Database (searchable):
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfrl/listing.cfmo. Electronic Product Radiation Requirements:
http://www.fda.gov/cdrh/comp/eprc.htmlp. Single-Use Device Reprocessing:
http://www.fda.gov/cdrh/reuse/index.htmlq. Guidance for Industry and for FDA Staff. Enforcement Priorities for Single-Use Devices Reprocessed by Third Parties and Hospitals:
http://www.fda.gov/cdrh/reuse/1168.htmlr. Product Code Classification Database (searchable): http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/pcdsimplesearch.cfm
s. Good Guidance Practices Database (searchable):
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfggp/search.cfm
IMPORTANT NOTE: The following MDR requirements have been stayed or revoked:
This document provides general guidance regarding the reporting of adverse events required by the Medical Device Reporting (MDR) Regulation.
A. PER SE RULE
This requirement no longer exists. Therefore, the submission of an event by a health care professional does not require the manufacturer to report the event based solely on the statements of a health care professional. The event must meet the reporting criteria in MDR to qualify as a reportable event.
B. REPORTING TIME FRAMES
Firms now have up to 30 CALENDAR days after they become aware of a device related death, serious injury or malfunction before they are required to submit a report to FDA.
C. FIVE-DAY REPORTS
Five-day reports are required in two circumstances. First, they are required if a manufacturer becomes aware that a reportable event, from any source of information, necessitates remedial action to prevent an unreasonable risk of substantial harm to the public health. Second, five-day reports are required when a manufacturer becomes aware of an MDR reportable event for which FDA has requested a five-day report.
D. NON-REPORTABLE EVENTS
Firms must submit MDR reports when the reported information reasonably suggests an association between one of its devices and a reportable death, serious injury or malfunction. Under some circumstances, an adverse event may appear to trigger the requirement of submission of an MDR, but because information reveals the device did not cause or contribute to the death or serious injury, no MDR is required. Thus as described below, a manufacturer will have to investigate the event in order to know if it should be reported.
A firm is required to submit an MDR report when it becomes aware of information reasonably suggesting that an event meets the criteria for reporting a Death, Serious Injury, or Malfunction. For example, a hospital informs a manufacturer that its device has failed and, as a result, a patient died. At this point, the firm has become aware of information that reasonably suggests they are in receipt of a reportable MDR event.Next, the firm must investigate the report to determine its cause. Both the QS Regulation and MDR require investigation of complaints. During its investigation a firm may become aware of information that changes the initial report's conclusions. For example, the firm may find that its device was not involved in the death and could not have caused or contributed to the death. In these instances the firm would document the information that changes the association between its device and the death. No report would be required if the death or other facts turn out to be incorrect. But, if the firm becomes aware of the identity of the device/firm that was associated with the death, the firm is responsible for forwarding the information to the FDA.
However, if the firm's investigation does not change the alleged association between the device and the death, the event must be submitted as an MDR report. In addition, if the firm's investigation produces information that would cause a person who is qualified to make a medical judgment to reach a reasonable conclusion that the device did not cause or contribute to a reportable MDR event - no report is required. Translation - if a firm decides NOT to report an apparent device-related death, serious injury or malfunction - this decision must be made by a person that the regulation recognizes as qualified to make a medical judgment, i.e., a physician, nurse, risk manager, or biomedical engineer. Using the example from above, if the firm's investigation yields an autopsy finding that the patient died from cancer – not the device - the firm could decide NOT to report as long as the decision is consistent with the regulation:
- There is documented information that changes the association between the death and the device,
- The decision is made by a person who is qualified to make a medical judgement, and
- The conclusion reached by the person in item two is reasonable.
PLEASE NOTE THE FOLLOWING:
- Firms ARE NOT required to have every MDR report reviewed by a person qualified to make a medical judgement and/or a person with a medical degree or training. Individuals who are not qualified to make a medical judgement can review MDR reports and make decisions on the basis of facts but they cannot make decisions NOT to report MDR events that require medical judgement.
- In lieu of in-house or on-site qualified medical personnel or individuals qualified to make a medical judgement the firm may use consultants.
- When reviewing a non-reportable event validate and document the credentials of the individual making these decisions as well as the decision not to report the event.
E. INVESTIGATION
Firms are required to investigate EVERY device related death, serious injury and malfunction in accordance with QS regulation, 820.198. Failure to comply with this provision is a violation of BOTH the QS regulation and MDR. Manufacturers are also required to VERIFY information on each form FDA 3500A as well as make a good faith effort to obtain information that is missing/not provided by the reporter. If the firm cannot obtain the missing information, the MDR complaint files shall contain an explanation of why the information could not be obtained as well as documentation of the firm's efforts to obtain the missing information.
F. REASONABLY KNOWN INFORMATION
FDA considers information that can be obtained by contacting the reporter to be in the possession of a firm, and considers information that can be obtained by analysis, testing, or other evaluation of a device to be information that a firm is expected to REASONABLY know, obtain and report.
G. REASONABLY KNOWN/GOOD FAITH EFFORT
A firm must demonstrate that it exercised "good faith" in any failed attempts to obtain required data that is missing, incorrect, or that FDA considers to be reasonably known. While the concept of good faith is generally considered to be equivalent to "due diligence", CDRH has not developed a standard. However, the firm's procedures for obtaining missing information should appear under the "Internal Systems" section of its written MDR procedures. In addition, the Center believes that the parameters of good faith effort must, at a minimum, comport with the level of risk/nature of the device associated with the event being investigated.
H. SERIOUS INJURY
The interpretation of what constitutes a serious injury can be subjective and complicate the enforcement of MDR. The "unanticipated temporary impairment" part of the former serious injury definition has been rescinded, thus alleviating a source of subjectivity. In addition, the requirements that intervention be "immediate" and the concept of "probability" have also been removed from the serious injury definition.
The current MDR regulation states that a serious injury is an “injury or illness." This literally means that there has to be an injury that is life-threatening, results in permanent impairment/damage, or necessitates medical/surgical intervention to preclude permanent impairment/damage in order for an event to be reportable as a serious injury. If there is no injury attributable to the device, then there is no serious injury report, however, the event may qualify as an MDR reportable malfunction depending upon the circumstances.
The Center may decide to clarify the definition of serious injury. These categories will be provided to the field and the industry through MDR guidance documents and/or letters, as necessary.
I. MALFUNCTIONS
Malfunction reporting decisions have been the subject of concern by both industry and the FDA. Basically, a malfunction is an event that is likely to cause or contribute to either a death or serious injury, but some circumstance prevented the injury or death from occurring. These events are very important since they represent "potential" deaths or serious injuries and provide the Agency with the opportunity to be proactive in reducing risks. Not all malfunctions, however, are MDR reportable events.
If a malfunction is not reportable as an MDR, it may be a complaint and thus subject to the QS complaint handling requirements. Determining if an event is a reportable malfunction involves answering a number of questions including:
- Is the event device-related?
- Has the device failed to perform its intended function or meet its performance specifications?
- Is this failure likely to cause or contribute to a death or serious injury if the event were to happen again?
There is a presumption in the MDR regulations that if the event happened once it can happen again. The determination of whether to submit a report should be based on the potential outcome. For example, if this malfunction were to occur, how would it affect the patient? If the answer is "the malfunction is likely to cause or contribute to death or serious injury" then the event is reportable. The preamble to the MDR regulations (Federal Register: December 11, 1995, Volume 60, Number 237, pages 63577-63607) offers the following guidance for determining circumstances in which malfunctions should be reported
- The chance of a death or serious injury occurring as a result of the recurrence of the malfunction is not remote;
- The consequences of the malfunction affect the device in a catastrophic manner that may lead to a death or serious injury;
- The malfunction results in the failure of the device to perform its intended essential function and compromises the device's therapeutic, monitoring or diagnostic effectiveness, which could cause or contribute to a death or serious injury.
NOTE: The essential function of a device refers, not only to the device's labeled use, but for any use widely prescribed within the practice of medicine.- The malfunction involves a long-term implant or a device that is considered to be life-supporting or life-sustaining and thus is essential to maintaining human life. Malfunctions of long-term implants are not routinely or "automatically" reportable unless the malfunction is likely to cause or contribute to a death or serious injury if it recurs.
- The manufacturer takes or would be required to take an action under sections 518 or 519(f) of the Act as a result of the malfunction of the device or other similar devices.
Conversely, malfunctions ARE NOT REPORTABLE if they are not likely to result in a death, serious injury, or another malfunction.
Food and Drug Administration
Center for Devices and Radiological Health
PO Box 3002
Rockville, MD 20847-3002
NOTE: Envelopes must be specifically identified with the type of report enclosed, e.g., Manufacturer Report, User Facility Report, Baseline Report, Annual Report, Five-Day Report, Supplemental Report, etc.,
http://www.fda.gov - once connected select the CDRH icon.
http://www.fda.gov/cdrh - CDRH home
STANDARD OPERATING PROCEDURES
Each device manufacturer and importer shall submit a written report to FDA of any correction or removal of a device IF the correction or removal was initiated to:
a) Reduce a risk to health posed by the device; or
b) Remedy a violation of the act caused by the device which may present a risk to health.
c) Reports in items (a) and (b) above are NOT required IF:
i. The information has already been reported to FDA under the MDR regulation, 21 CFR 803 or under 21 CFR 1004.
NOTE: The MDR report must:
- Be submitted within the 10 day reporting timeframe specified in 21 CFR 806.10, and
- Contain all the information required in a Report of Correction and Removal as specified in 21 CFR 806.10(c)(1-13).
ii. The correction or removal meets the following criteria:
- When the action is taken to improve the performance or quality of a device but does not reduce a risk to health posed by the device or remedy a violation of the act caused by the device.
- Market withdrawals, 21 CFR 806.2(h) and 21 CFR 7.3(j) - a correction or removal of a distributed device that involves a minor violation of the act that would not be subject to legal action by FDA or that involves no violation of the act, e.g., normal stock rotation practices.
- Routine servicing, 21 CFR 806.2(k) - any regularly scheduled maintenance of a device, including the replacement of parts at the end of their normal life expectancy, e.g., calibration, replacement batteries, and responses to normal wear and tear. However, repairs of an unexpected nature, replacement of parts earlier than their normal life expectancy or identical repairs or replacement of multiple units of a device are not routine servicing. Such service should be “trended” to determine if a problem exists.
- Stock recoveries, 21 CFR 806.2(l) and 21 CFR 7.3(k) - the correction or removal of a device that has not been marketed or that has not left the direct control of the manufacturer, i.e., the device is located on the premises owned, or under the control of, the manufacturer, and no portion of the lot, model, code, or other relevant unit involved in the corrective or removal action has been released for sale or use.
d) The key concept for determining when an event is reportable is the definition of risk to health found in 21 CFR 806.2(j):
i. A reasonable probability that use of, or exposure to, the product will cause serious adverse health consequences or death; (Class I Recalls) or
ii. That use of, or exposure to, the product may cause temporary or medically reversible adverse health consequences, or an outcome where the probability of serious adverse health consequences is remote, (Class II Recall).
NOTE: Assistance regarding risk to health determinations can be obtained from your district's recall coordinator or CDRH's recall staff in the Office of Compliance.
e) Manufacturers and Importers are required to submit a Corrections and Removals report to the appropriate FDA District Office within 10 working days of the decision to initiate a correction. A list of the information required in the report is listed in 21 CFR 806.10(c)(1-13).
f) A foreign manufacturer or owner or operator of devices must also submit reports of corrections and removals.
NOTE: The regulation does not specify where foreign device manufacturers should send their Corrections and Removals reports. FDA, however, expects foreign Corrections and Removals reports to be submitted to the District Office where the product is being imported.
a) Each device manufacturer and importer who initiates a correction or removal of a device that is NOT required to be reported to FDA under Section 806.10 shall keep a record of each correction or removal.
b) Records of corrections and removals NOT reported to FDA must contain the following information:
i. The brand name, common or usual name, classification name, product code (if known), and the intended use of the device.
ii. The model, catalog, or code number of the device and the manufacturing lot or serial number of the device or other identification number.
iii. A description of the event(s) giving rise to the information reported and the corrective or removal action that has been, and is expected to be taken.
iv. Justification for NOT reporting the correction or removal action to FDA, which shall contain conclusions and any follow-ups, and be reviewed and evaluated by a designated person.
v. A copy of all communications regarding the correction or removal.
c) Manufacturers shall retain all records required under this section for a period of 2 years beyond the expected life of the device, even if the respective firm has ceased to manufacturer or import the devices.
In addition, Corrections and Removal files/records must be transferred to any new/subsequent manufacturer or importer of the device and maintained for the required period of time.
1. Title 21 CFR Part 806, Medical Devices; Reports of Corrections and Removals.
2. Title 21 CFR Part 7, Enforcement Policy, (Recalls (Including Product Corrections)---Guidelines on Policy, Procedures, and Industry Responsibilities.
3. Title 21 CFR Part 803, Medical Device User Facility and Manufacturer Reporting.
Updated June 15, 2006
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH