CBER Presentation
FDA HCT/P Inspection Experience: Analysis by Area and General Update
3rd Annual FDA and the Changing Paradigm for HCT/P Regulation
January 24, 2007
Mary Malarkey, Director, OCBQ, CBER
Time Flies...20 months
Summary
- Inspections and actions by the numbers
- Regulatory action citations
- Specific citations by area
- Reproductive
- Hematopoietic stem/progenitor cells
- Recovery other than BTS and DRS
- Other
- Recalls
- BTS and DRS Update
- HTTF Update
FY06 HCT/P Inspections Accomplished
| Type of HCT/P establishment | # Inspections Accomplished | Hours/Inspection |
|---|---|---|
| Reproductive tissues | 87 | 45.7 |
| Cord blood stem cells Peripheral blood stem cells |
36 | 42.8 |
| All other HCT/Ps (e.g. musculoskeletal, ocular, recovery, distributors) | 234 | 44.1 |
| Total | 354* |
*Sum of individual inspections do not equal total (354) due to two inspections that were conducted for products in multiple categories
FY06 HCT/P Inspection Classifications
| Type of HCT/P establishment | NAI | VAI | OAI |
|---|---|---|---|
| Reproductive tissues | 55 | 26 | 5 |
| Cord blood stem cells Peripheral blood stem cells | 24 | 11 | 0 |
| All other HCT/Ps (e.g. musculoskeletal, ocular, recovery, distributors) | 170 | 59 | 5 |
| Total | 249 | 96 | 10 |
FY06 HCT/P Inspection Results
- Less than 30% of HCT/P inspections resulted in issuance of Form FDA-483s
- Regulatory Actions Issued in FY06
- 2 Orders to Cease Manufacture and Retain HCT/Ps - Recovery establishments
- 1 Warning Letter - Reproductive
- 2 Untitled Letters - Reproductive
Establish and Maintain?
- 1271.3(cc) - Establish and maintain means define, document (in writing or electronically, and implement; then follow, review, and, as needed, revise on an ongoing basis.
FY06 HCT/P Regulatory Actions Common Deviations
- Failure to establish and maintain procedures [1271.47(a) and 180(a)]
- Examples of procedures involved:
- Donor eligibility determination
- Donor screening
- Donor testing
- Plasma dilution
- Recovery
- Failure to establish and maintain accurate records [1271.55(d) and 270(a)]
- Examples of records involved
- Medical evaluation forms
- Physician interviews
- Next-of-kin interviews
- Recovery records
- Inadequate screening for risk factors/clinical evidence of communicable disease [1271.75(a)]
- Acceptance of product from ineligible donors [1271.50]
- Failure to recover in a way that does not cause contamination or cross-contamination [1271.215]
- Donor Testing Deviations:
- Not testing for communicable diseases [1271.85]
- Not using FDA licensed/approved/cleared kits [1271.80(c)]
- Incorrect timing of specimen collection [1271.80(b)]
FY06 Inspectional Observations: Reproductive
- Inadequate screening for risk factors/clinical evidence of communicable disease [1271.75(a)]
- Failure to follow procedures for all steps in donor screening and DE determination [1271.47(a)]
- Not testing for communicable diseases [1271.85(a) and (c)]. Donors determined eligible and product released though testing not completed for:
- anti-HIV-1/2
- anti-HCV
- Chlamydia trachomatis
- Neisseria gonorrhea
Reproductive
- Acceptance of product from ineligible donors [1271.50], as with last bullet on preceding slide
- Not using FDA licensed/approved/cleared kits [1271.80(c)]
- Incorrect timing of specimen collection [1271.80(b)]
FY06 Inspectional Observations: Hematopoietic Stem Cells
- Procedures [1271.180 and 47]
- Procedures not established
- Processing
- Labeling control
- Storage/Distribution
- Handling of positive test results
- Procedures not followed
- Donor screening
- Quality Program [1271.160]
- No quality program established
- Quality program does not ensure:
- Documentation of corrective actions
- Investigation and trending of HCT/P deviations
- Equipment [1271.200]
- Procedures for equipment cleaning, sanitization, and maintenance not established
- Equipment not cleaned, sanitized, and maintained according to schedule
- Supplies and Reagents [1271.210]
- Supplies and reagents not verified
- No records of receipt of supplies and reagents
- Records [1271.270]
- Records do not identify person performing work
- Donor Testing [1271.80]
- FDA licensed/approved/cleared tests not used
- Manufacturer's instructions not followed
FY06 Inspectional Observations: Recovery
- Quality Program [1271.160]
- Quality audits not performed periodically
- Quality program did not ensure that appropriate corrective actions taken and appropriate (donor screening deficiencies)
- Facilities [1271.190]
- Facilities not clean, sanitary, orderly
- Procedures for cleaning and sanitation of recovery sites not established and maintained
- No documentation of cleaning and sanitation of recovery sites
- Equipment [1271.200]
- Procedures for cleaning and maintenance of recovery equipment not established and maintained
- Procedures for calibration of measuring equipment (i.e. temperature monitoring) not established and maintained
- No documentation of cleaning and maintenance of recovery equipment
- No procedures for proper packaging and shipment [1271.265(e)]
- No procedures for complaints [1271.320(a)]
- Complete answers to donor's medical and social history not always documented [1271.55(d)(2)]
Other
- Ocular
- Facilities 1271.190
- Equipment 1271.200
- Processors
- Summary of records - 1271.55(b) - incomplete information
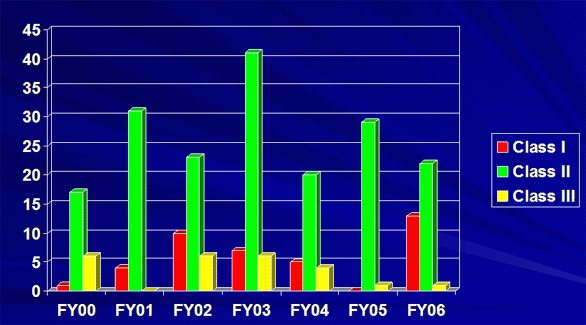
Classified Recalls FY 2006
| HCT/Ps | CBER Total (all products) | |
|---|---|---|
| FY 06 Class I | 13 | 13 |
| FY 06 Class II | 22 | 1377 |
| FY 06 Class III | 1 | 451 |
FY 2006 Class I HCT/P Recalls
- Biomedical Tissue Services (BTS), Ft. Lee, New Jersey
- HCT/Ps, recovered from donors without adequate donor eligibility determinations, were distributed.
- Recalled HCT/Ps collected from 761 donors
- Subrecalls performed at several processors
- 9 of 13 Class I recall numbers related to HCT/Ps recovered by BTS
FY 2006 Class I HCT/P Recalls
Other Reasons for Recall
- Companion tissue + for microorganisms
- Recipient of companion tissue reported infection with Clostridium septicum
- Donor HCV NAT + (firm informed after distribution)
- Donor HCV + by EIA and NAT (results attributed to wrong donor)
HCT/P Recalls Classified
Biomedical Tissue Services (BTS) Order to Cease and Retain HCT/Ps
- Immediately cease manufacturing operations and retain HCT/Ps.
- To BTS and its CEO and Executive Director, Michael Mastromarino, D.D.S.
- After initially focusing efforts on assessing the safety of distributed tissue and facilitating recalls, FDA determined that the violations uncovered at BTS, because of their serious nature, constitute a danger to health and took this unprecedented action
- Order to Cease Manufacturing and to Retain HCT/Ps issued January 31, 2006
- www.fda.gov/cber/compl/bts013106.htm
Updated Notification
- Issued March 2, 2006 www.fda.gov/cber/safety/bts030206.htm
- New information that calls into question the reliability of some of the donor blood samples submitted for communicable disease testing from BTS tissue donors. In some instances, blood samples did not come from the same donor as the linked tissues
- Results of communicable disease tests obtained from the blood sample may not correctly reflect the status of the donor
CDC: MMWR Brief Report May 26, 2006
- "Investigation into Recalled Human Tissue for Transplantation---United States, 2005--2006."
- Approximately 25,000 BTS-recovered tissue products distributed to all 50 states and internationally
- FDA and CDC continue to investigate reports of BTS recipients who have undergone screening and tested positive for one of the four tested diseases
- Some positive results would be expected in any U.S. population tested (prevalence data provided)
- Relationship between implanted BTS tissue and positive test results reported to FDA and CDC is difficult to ascertain because of inaccurate BTS donor records and, in some cases, the absence of properly linked donor samples.
Donor Referral Services (DRS) Order to Cease and Retain HCT/Ps
- Immediately cease manufacturing operations and retain HCT/Ps.
- To DRS and its Owner, Philip Guyett
- FDA determined that the violations uncovered at DRS, because of their serious nature, constitute a danger to health.
- Order to Cease Manufacturing and to Retain HCT/Ps issued August 18, 2006
- www.fda.gov/cber/compl/drs081806.htm
DRS Order
- The order alleges that FDA's inspection uncovered serious violations of the regulations governing donor screening and record-keeping practices, and:
- failure to create and maintain accurate records;
- failure to establish and maintain SOPs for all steps performed in manufacturing of HCT/Ps;
- failure to review relevant medical records regarding risk factors for, or clinical evidence of, relevant communicable disease agents and diseases.
- FDA found numerous instances where information provided by DRS to a tissue processor was at variance with the death certificates FDA obtained from the state where the death occurred:
- Cause, place, and time of death
- FDA continues to investigate DRS working with other federal, state and local authorities
DRS Public Health Notification
- August 30, 2006
- FDA and CDC strongly recommending health care providers inform all patients that received tissue initially recovered by DRS and offer access to communicable disease testing
- Distributing firms had already voluntarily recalled tissue in inventory
- Where FDA had previously identified specific cases of concern, the firms cooperated fully in efforts to inform patients and offer testing in those cases
Human Tissue Task Force
- Formed in August to evaluate effectiveness of new regulations and to identify whether additional steps necessary to strengthen regulatory framework
- Collaborative effort between CBER, ORA and OC
- Extremely productive three months
- Initial recommendations provided to CBER and ORA senior management
We're Here to Help You!
- Email CBER:
- Manufacturers: matt@cber.fda.gov
- Consumers, health care
- Phone:
- +1-301-827-1800
octma@cber.fda.gov


