CBER Presentation
FDA Update: Relevant to Reproductive Establishments
Martha A. Wells, MPH, RAC
Division of Human Tissues
Office of Cellular, Tissue, and Gene Therapies
Center for Biologics Evaluation and Research (CBER), FDA
martha.wells@fda.hhs.gov
American Association of Tissue Banks
Annual Meeting
September 17, 2007, Boston, MA
Will Discuss
- FDA Requirements: History/Applicability
- Registration and Listing
- Donor Eligibility (DE) Requirements
- Current Good Tissue Practice (CGTPs)
- Testing Guidance –Pooled specimens for Nucleic Acid Tests (NAT) / diagnostic tests
- Recently posted DE Guidance
- Specific donor eligibility questions
- Inspectional information
21 CFR Part 1271
| Regulation Subparts | Proposed | Final | Effective Date |
|---|---|---|---|
| Establishment Registration and Product Listing | 1998 | 2001 | 2001 for establishments regulated under 1270 2004 for newly regulated establishments |
| Donor Eligibility | 1999 | 2004 | May 25, 2005 |
| Current Good Tissue Practice (CGTP): Inspection and Enforcement | 2001 | 2004 | May 25, 2005 |
Applicable FDA Requirements for Reproductive Establishments
- Registration and listing if the establishment performs any manufacturing function and doesn’t fit any of the 1271.15 exemptions
- Donor eligibility
- Required for donors of semen and oocytes if anonymous or directed
- Recommended for donors of embryos if both gametes were from the sexually intimate partner
- Current Good Tissue Practice –only parts applicable
- 1271.150(c ) manufacturing arrangements
- 1271.155 exemptions and alternatives
- Inspections and enforcement
Registration Form FDA 3356
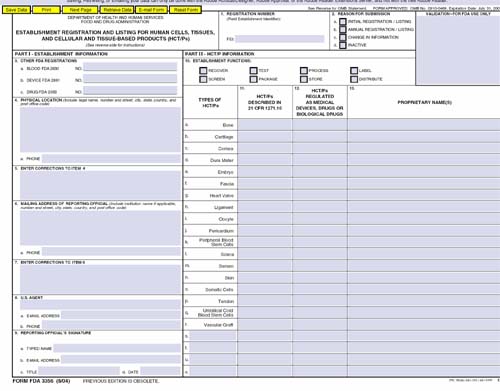
Registration and Listing
- Annual update required in December
- Electronically registered establishments were notified via email last year
- Electronic registration is encouraged
- 63% did so last year
- Current Form 3356 expired 7/31/07 – can still use to make changes
- Changes to Form 3356 being considered
- For December annual registration
- To capture more information about the HCT/Ps listed
- Assist in planning inspections
Registration and Listing
- If you do not perform donor infectious disease testing in house – do not check test – over 500 establishments indicate they test
- Changes to HCT/P listing are required within 6 months
- Change of ownership or location required within 5 days
- Inactivation— check box #2(d)

Failure to Register
- If you do not renew your annual registration CBER’s Office of Compliance will contact your FDA District Office for follow-up
- Information on failure to register has been posted on CBER’s website www.fda.gov/cber/tissue/failreg.htm
- Registration Information: www.fda.gov/cber/tiss.htm
- Questions: Questions: tissuereg@fda.hhs.gov
Registration and Listing
|
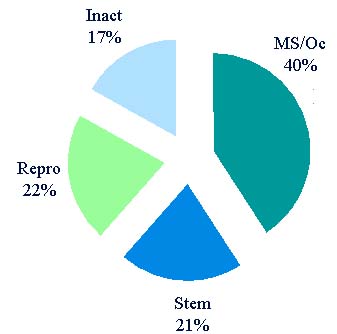 |
Registration and Listing
- 685 establishments list reproductive HCT/Ps
- 88 registered that only list semen
- Cryobanks – anonymous donors
- Andrology labs store semen for procedures forintimate couples
- 30 registered that only list oocytes
- Banks that store oocytes
- Matching agencies
- Registration of establishments that only administer donor history questionnaires, but do not determine donor eligibility - currently under discussion
CBER/CDER Proposed Registration & Listing
Rule: Foreign and Domestic Establishments
(would affect Part 1271)
- Published August 29, 2006
- Currently working on a draft of the final rule
- New definitions: importer, US agent
- Would require electronic registration unless a waiver is granted
- Foreign establishments importing HCT/Ps would submit information on all known US consignees
- Changes to ownership or location would be reported within 30 days rather than 5 days
Donor Eligibility Determination
- Required for anonymous/directed donors
- Procedures/records required
- Summary of records must accompany the HCT/P
- Collection of blood specimen within certain time periods before or after recovery
- Required screening –within a reasonable time period before or after recovery
- Donor medical history interview
- Physical examination
Donor Eligibility - Semen
- Anonymous –6 month cryopreservation in quarantine with negative re-test/screen before release
- Directed/known –no quarantine
- Recent screen (interview and physical exam)
- Collect blood specimen within 7 days before recovery
- If test/screen positive the donor is ineligible but can be used with physician notification, and labeling
- DE is required if the intent is to use a gestational carrier/surrogate
Donor Eligibility -Oocytes
- Anonymous
- Recent negative screening
- Collect blood specimen within 30 days before recovery
- Directed/Known
- Recent screen (interview and physical exam)
- Collect blood specimen within 30 days before recovery
- If test/screen positive –ineligible but still can be used with physician notification and labeling
- DE is required if intent is to use a gestational carrier/surrogate
Exceptions: Donor Eligibility Not Required
- For reproductive cells or tissues donated by a sexually intimate partner (SIP) of the recipient for reproductive use (1271.90(a)(2))
- For cryopreserved cells or tissue for reproductive use, other than embryos, exempt at the time of donation (autologous or SIPs) that are subsequently intended for directed donation (1271.90(a)(3)) provided that:
- Additional donations are unavailable, for example, due to the infertility or health of a donor; and
- Appropriate measures are taken to screen and test the donor(s) before transfer to the recipient
Exceptions: Donor Eligibility Not Required
- For cryopreserved embryos from SIPs and subsequently intended for donation (1271.90(a)(4)):
- Anonymous or directed
- To another couple or individual
- To a gestational carrier
- Test/screen recommended at time of donation.
- Special labeling required (if not screened/tested at time of donation)
- If a qualified 3rd party donor is used – embryos can still be donated
- Note: DE is required for SIP gamete donors intending to form an embryo for use in gestational carrier
Required Labeling for Exceptions
1271.90(b)
- Must be on container or accompanying tissue
- “NOT EVALUATED FOR INFECTIOUS SUBSTANCES”unless all applicable screening and testing performed (not if (b)(6) label used)
- “WARNING: Advise recipient of communicable disease risks”if:
- DE determination not performed or not completed; or
- Results of screening or testing positive.
- Not applicable to autologous tissues
Required Labeling for Exceptions
1271.90(b)
- Use the Biohazard legend if results of screening or testing are positive
- “WARNING: Reactive test results for (name of disease agent or disease)”in the case of reactive test result
- “Advise recipient that screening and testing of the donor(s) were not performed at the time of cryopreservation of the reproductive cells or tissue, but have been performed subsequently for 1271.90 (a)(3) or (4)
CGTP Requirements for Reproductive Establishments
- Manufacturing arrangements -1271.150(c)
- Ensure that an establishment (e.g., testing lab) that performs a step in manufacture for you (under contact, agreement, or other arrangement) is in compliance with applicable requirements
- Guidance published – September 2006
- Exemptions and Alternatives -1271.155
- From DE or CGTP requirements
- Must be consistent with the goals of protecting the public health and/or preventing the introduction, transmission, or spread of communicable diseases
Guidance: HCT/Ps Tested Using Pooled Specimens or Diagnostic Tests
- Published 1/23/2007 for immediate implementation
- Applicable to certain HCT/Ps from living donors recovered after 5/25/2005 to 30 days after publication
- Addresses:
- Distributed HCT/Ps and those in inventory
- Need for HCT/P deviation reports if distributed
- Retesting/labeling for future distribution of HCT/Ps in inventory
Testing Guidance, continued
- Limited to two testing situations
- Used a diagnostic test when a donor screening test available
- Performed Nucleic Acid Testing (NAT) using pooled specimens rather than individual donor specimens
- Pertains to recently regulated living donors of HCT/P (hematopoietic stem/progenitor cells and reproductive cells and tissues)
- HCT/P deviation reports not required for reproductive cells and tissue (only for hematopoietic stem cells from first or second degree blood relatives)
Testing Guidance: Donor Retesting
- If retesting is performed, FDA is exercising enforcement discretion for distribution/ release from inventory
- Recommends using original donor specimen
- New specimen OK
- Tested in accordance with the manufacturer’s instructions and found negative/nonreactive
- If retesting is not feasible, because
- Retained specimen is not available; or
- Donor cannot be located or refuses to be retested, then…
Testing Guidance: Donor Retesting Not Feasible: Cryopreserved Embryos
- Formed for SIPs using a 3rd party gamete donors can be distributed (FDA is exercising enforcement discretion)
- Documentation in accompanying records and in files why retesting not feasible
- Physician notification
- Labeled “WARNING: Advise patient of communicable disease risks”
- If pooled NAT was used –list NAT test used and result in summary of records and add qualifier, “not performed according to the manufacturer’s instructions”
- If diagnostic test was used—list diagnostic test performed and result in summary of records and add qualifier, “diagnostic tests used, instead of donor screening tests”
Testing Guidance: Donor Retesting Not Feasible
- Cryopreserved semen or oocytes from anonymous or directed donors
- If retesting is not feasible,
- HCT/Ps must not be distributed
Donor Eligibility Guidance
- Reposted on August 8, 2007
- Replaces version of the guidance posted February 27, 2007
- Recommendations should be implemented as soon as feasible, but not later than August 27, 2007
- Only technical/typographical changes made
- Dr. Greenwald will go into more detail on Tuesday
DE Guidance: Changes Relevant to Reproductive Tissues, Clarifies That:
- Only viable, leukocyte-rich donors should be evaluated for clinical evidence of HTLV
- If you test for HIV-group O, you do not have to ask the screening questions concerning residence/blood transfusion in Africa
- FDA cleared diagnostic serological tests for syphilis can be utilized
- Persons who have received human derived clotting factor concentrates only once may be eligible to donate
- A medical record can serve as evidence of treatment for chlamydiaand gonorrhea treatment. Donor can be used if at least 12 months since treatment and a current test is negative.
Donor Eligibility Questions
- Do I have to test all donors for CMV and HTLV?
(No, only leukocyte rich HCT/Ps –semen) - My donor lived in Europe for more than 5 years, may I accept her to donate?
(Only for directed donation) - May I use a donor who had chlamydia, gonorrhea or syphilis and was treated?
(Yes, if disease and documented that treatment occurred more than 12 months ago and donor tests negative now)
Donor Eligibility Questions
- Do I have to perform a DE determination for the SIP donors when embryos were formed to be carried for the couple by a gestational carrier?
(Yes) - May cryopreserved embryos formed from an untested SIP and a qualified 3rdparty donor be donated?
(Yes) - May I use a donor that tests positive with an antibody screening test, but confirmatory test is negative?
(No, except if syphilis test)
Donor Eligibility Questions
- If a donor is tested after February 23, 2007 using pooled NAT or diagnostic tests where a screening test is available, may I release any cryopreserved HCT/Ps from this donor if I retest?
(No) - My donor recently had a tattoo, can she donate?
(Yes, unless sterile procedures were not used) - Do I have to screen and test a gestational carrier and her partner
(No)
Reproductive Establishment Inspections Accomplished Since May 25, 2005
- 205 inspections / 59 received listing of observation (29%)
- 45.7 average hours/inspection
- 2 warning letters
- 3 untitled letters
Top 10 Inspectional Observations in Reproductive Establishments
- 1271.47(a) DE Procedures (30)
- Not established, maintained, documented, reviewed or revised
- Not established for all donor eligibility steps
- Not designed to ensure compliance with the donor eligibility requirements
- 1271.50(a) DE determination based on screening and testing (18)
- Not performed
- Not determined by a responsible person based on results of donor screening and testing
Top 10 (# of observations)
- 1271.75(a) Risk factors, clinical evidence (11)
- Donors not screened by a review of relevant medical records for risk factors, clinical evidence
- 1271.80(b) Specimen collections not timely (10)
- 1271.75(e) Abbreviated procedures (9)
- Used for donors who had no complete screening in the previous six months
- Did not determine and document changes in the donor’s medical history that would make the donor ineligible
Top 10
- 1271.47(b) Review and approval of procedures not performed by a responsible person before implementation (8)
- 1271.80(c) Test kits used were not FDA approved for donor screening or manufacturer instructions were not followed (8)
- 1271.85(c) Reproductive cells or tissues not recovered by a method that ensures freedom from contamination were not tested for genitourinary tract communicable diseases (7)
- 1271.150(c)(1)(iii) Ensure compliance (5)
- For establishments performing manufacturing steps under contact, agreement or other arrangement
CBER CONTACT INFORMATION
- Manufacturers: matt@cber.fda.gov
- Consumers, health care professionals: octma@cber.fda.gov
- Phone: 800-835-4709 or 301-827-1800
- Web: www.fda.gov/cber/tiss.htm


