CBER Presentation
FDA Adverse Reaction Reporting System for Human Cells, Tissues and Cellular and Tissue-Based Products (HCT/Ps)
Martha A Wells, MPH
Office of Cellular, Tissue, and Gene Therapies
Center for Biologics Evaluation and Research (CBER), FDA
WHO Meeting, June 2006
FDA's Reporting Requirement: 21 CFR Part 1271.350-Effective May 25, 2005
- Manufacturers must investigate:
- Any adverse reaction involving a communicable disease related to an HCT/P that they made available for distribution.
- Manufacturers must report to FDA
- An adverse reaction involving a communicable disease if it:
- Is fatal
- Is life-threatening
- Results in permanent impairment of function or permanent damage to body structure; or
- Necessitates medical or surgical intervention, including hospitalization.
FDA's Reporting Requirements
- Adverse reaction means a noxious and unintended response to any HCT/P for which there is a reasonable possibility that the HCT/P caused the response
- To report adverse reactions to FDA, manufacturers must submit a MedWatch 3500A to FDA within 15 days of receipt of information
- And provide follow-up within 15 days of receipt
How are Adverse Reactions Reported to FDA?
- For Manufacturers:
- Use Form FDA 3500A (Medwatch)
- Have 15 days from receipt of information
- For Voluntary reporters
- Use Form FDA 3500 (Medwatch)
- Also promptly report to HCT/P establishments
- FDA Medwatch reporting system
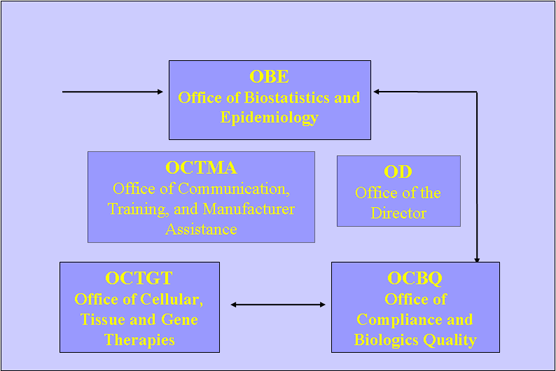
The Journey: MedWatch Form Triage

Tissue Safety Team (TST)
- Consists of CBER representatives from four CBER offices and the Center Director's Office
- Coordinates with CDC, FDA District Offices, Center for Devices, etc as needed
- Purpose is to coordinate responses to reports of HCT/P adverse reactions and to develop procedures to facilitate rapid and comprehensive responses by FDA
Tissue Safety Team
CBER's TST Follow-up on MedWatch Reports
- Occurs if report indicates an infectious disease transmission or possible transmission that may be associated with an HCT/P
- Involves gathering information from reporter or manufacturer, as indicated
- Tries to determine if the infection was transmitted by the tissue (difficult to determine in most cases)
TST to Date
- Guidance for Industry: Medwatch Form FDA 3500A: Mandatory Reporting of Adverse Reactions Related to HCT/Ps:11/30/2005
- CBER SOPP 8508: Procedures for Handling Adverse Reaction Reports Related to "361" HCT/Ps, 11/28/2005
- Developed Database for HCT/P Medwatch reports and follow-ups
- Additional information at http://www.fda.gov/cber/tissue/hctadverse.htm
- Approximately 70 follow-ups to date
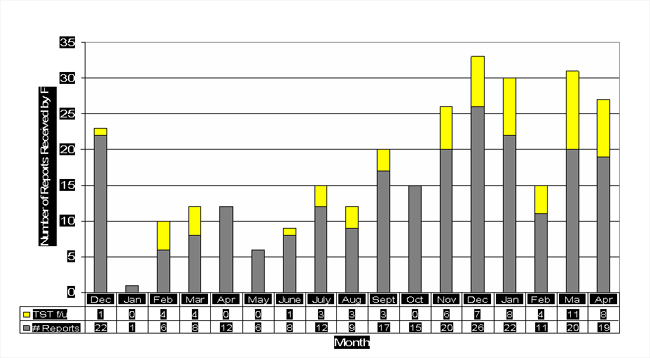
HCT/P Medwatch Reports Received by FDA 12/04-04/06
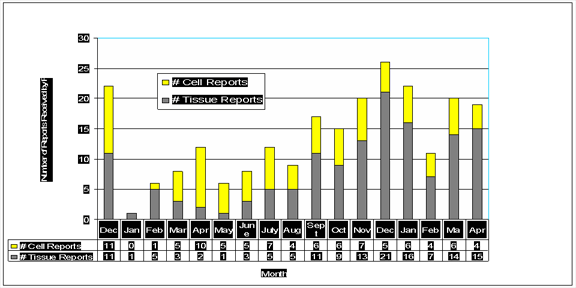
TST Follow-ups (12/04-4/06)
Adverse Reaction Reporting and HCT/P Safety
- Required reporting for HCT/P manufacturers
- With MedWatch, FDA can detect trends across the country that may not be identified at an individual site
- Goal of HCT/P surveillance is to prevent additional adverse reactions
CONTACT INFORMATION
Martha A. Wells, MPH
Chief, Human Tissues and Reproduction Branch
Division of Human Tissues, OCTGT
CBER/FDA
1401 Rockville Pike (HFM 775)
Rockville, MD 20852-1448
Martha.Wells@fda.hhs.gov
301-827-6106


