CBER Presentation
FDA Inspections
Tissue/Cell Establishments
Martha A Wells, MPH
Office of Cellular, Tissue, and Gene Therapies
Center for Biologics Evaluation and Research (CBER), FDA
WHO Meeting, June 2006
Inspections and Enforcement 21 CFR Part 1271, Subpart F
- Required by regulation
- Frequency at Agency discretion
- Generally unannounced
- Performed by trained investigators from FDA's District Offices
- Investigator may take samples, review and copy records
- List of observations generated if practices are not in compliance
Focus for FDA Inspections - Tissue Establishments
- Record review
- Donor eligibility determination -Screening and testing
- All manufacturing steps
- Standard operating procedure review
- For all steps in manufacture
- Focus on preventing circumstances that might increase risk of introduction, transmission, or spread of communicable disease
- Personnel and Training
- Quality program
- Facility issues
- Environmental control
- Storage/Equipment
- Supplies and reagents
- Labeling and labeling controls
- Processing - controls and validation
- Tracking
- Complaint files and tracking system
Enforcement Actions by FDA
- Can order establishment to recall, destroy or retain products
- Can take possession of and/or destroy the violative tissue/cells
- Can order establishment to cease manufacturing
- Other actions
- Untitled and warning letters
- Regulatory meeting with FDA
- Prosecution and fines
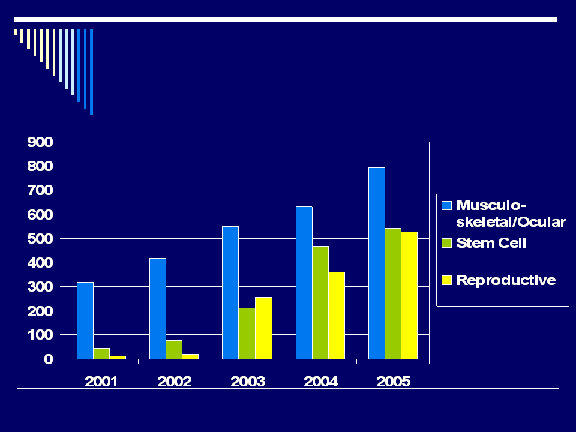
Registered Establishments
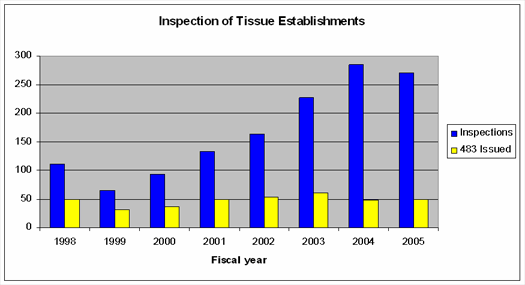
Tissue Inspections
Inspections: Role of Professional Associations
- Accreditation may be taken into consideration for FDA inspectional planning
- Professional association accreditation focused more on education than on compliance/enforcement - voluntary
- Development of standards and accreditation programs - may enhance establishment's probability of being in compliance with FDA requirements
- Associations assist in training FDA investigators
Third Party Inspections?
- Might be considered by CBER in the future for tissue establishments
- First need baseline inspectional history
- Other FDA models
- Mammography Quality Standards Inspections
- Inspection of certain facilities performed by some states under contract with FDA
- Device manufacturers inspection by FDA accredited persons
CONTACT INFORMATION
Martha A. Wells, MPH
Chief, Human Tissues and Reproduction Branch
Division of Human Tissues, OCTGT
CBER/FDA
1401 Rockville Pike (HFM 775)
Rockville, MD 20852-1448
Martha.Wells@fda.hhs.gov
301-827-6106


