 |
|
|
|
|
|
||||||||
|
||||||||||
|
|
||||||||||
| Home » About NHLBI » Director's Page » Budget and Legislative Information: Congressional Testimony |
DEPARTMENT OF HEALTH AND HUMAN SERVICES
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Source of Funding |
FY 2007 Actual |
FY 2008 Enacted |
FY 2009 Estimate |
|---|---|---|---|
| Appropriation | $2,921,757,000 | $2,974,900,000 | $2,924,942,000 |
| Pay cost add-on | 1,172,000 | 0 | 0 |
| Rescission | 0 | -51,972,000 | 0 |
| Subtotal, adjusted appropriation | 2,922,929,000 | 2,922,928,000 | 2,924,942,000 |
| Real transfer under Director's one-percent transfer authority (GEI) | -538,000 | 0 | 0 |
| Comparative transfer to NIBIB | -82,000 | 0 | 0 |
| Comparative transfer to OD | -37,000 | 0 | 0 |
| Comparative transfer to NCRR | -2,827,000 | 0 | 0 |
| Comparative
transfers to the Office of the Assistant Secretary for Admin. and Mgmt. and to the Office of the Assistant Secretary for Public Affairs |
-3,000 |
0 |
0 |
| Comparative transfer to NIDDK | -800,000 | -816,000 | 0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) | 538,000 | 0 | 0 |
| Subtotal, adjusted budget authority | 2,919,180,000 | 2,922,112,000 | 2,924,942,000 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | 2,919,180,000 | 2,922,112,000 | 2,924,942,000 |
| Unobligated balance lapsing | -68,000 | 0 | 0 |
| Total obligations | 2,919,112,000 | 2,922,112,000 | 2,924,942,000 |
(1)Excludes the following amounts for reimbursable activities carried
out by this account:
FY 2007 - $13,593,000 FY 2008 - $20,000,000 FY 2009 - $20,000,000
Excludes $1,400,000 in FY 2008 and $1,400,000 in FY 2009 for royalties.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FY 2005 Actual |
FY 2006 Actual |
FY 2007 Actual |
FY 2007 Comparable |
FY 2008 Enacted |
FY 2009 Estimate |
Change |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extramural Research | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Detail: | ||||||||||||||
| Heart and Vascular Diseases | 1,607,188,000 | 1,632,680,000 | 1,633,337,000 | 1,632,011,000 | 1,628,885,000 | 1,627,986,000 | -$899,000 | |||||||
| Lung Diseases | 631,725,000 | 600,892,000 | 601,134,000 | 600,646,000 | 599,496,000 | 599,164,000 | -$332,000 | |||||||
| Blood Diseases and Resources | 442,082,000 | 417,578,000 | 417,746,000 | 417,407,000 | 416,607,000 | 416,377,000 | -$230,000 | |||||||
| Subtotal, Extramural | 2,680,995,000 | 2,651,150,000 | 2,652,217,000 | 2,650,064,000 | 2,644,988,000 | 2,643,527,000 | -1,461,000 | |||||||
| Intramural research | 405 | 170,200,000 | 402 | 170,736,000 | 413 | 169,560,000 | 413 | 168,678,000 | 414 | 175,134,000 | 414 | 177,845,000 | 0 | 2,711,000 |
| Res. management & support | 391 | 90,006,000 | 395 | 97,864,000 | 401 | 100,614,000 | 401 | 100,438,000 | 401 | 101,990,000 | 407 | 103,570,000 | 6 | 1,580,000 |
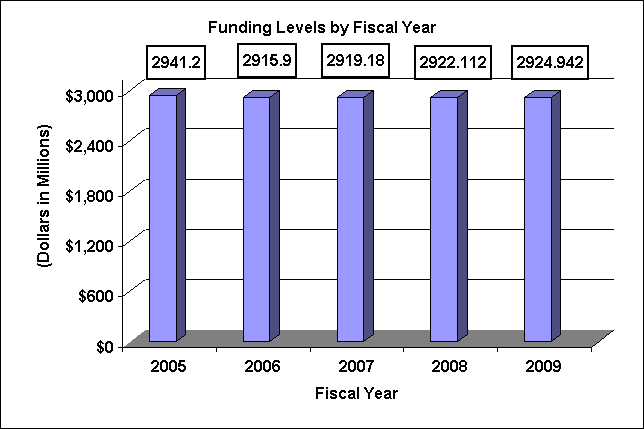
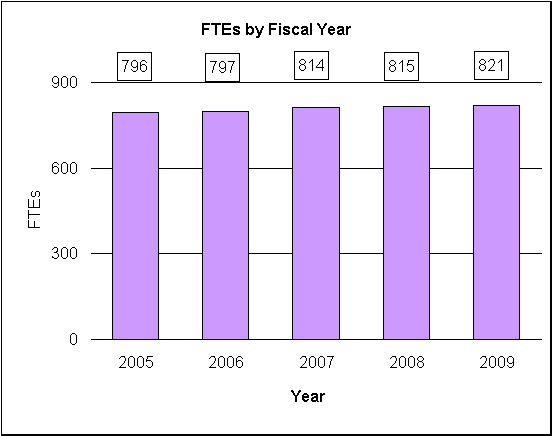
| TOTAL | 796 | 2,941,201,000 | 797 | 2,919,750,000 | 814 | 2,922,391,000 | 814 | 2,919,180,000 | 815 | 2,922,112,000 | 821 | 2,924,942,000 | 6 | 2,830,000 |
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2009 budget request for NHLBI, which is +$2.830 million more than the FY 2008 Enacted, for a total of $2,924.942 million.
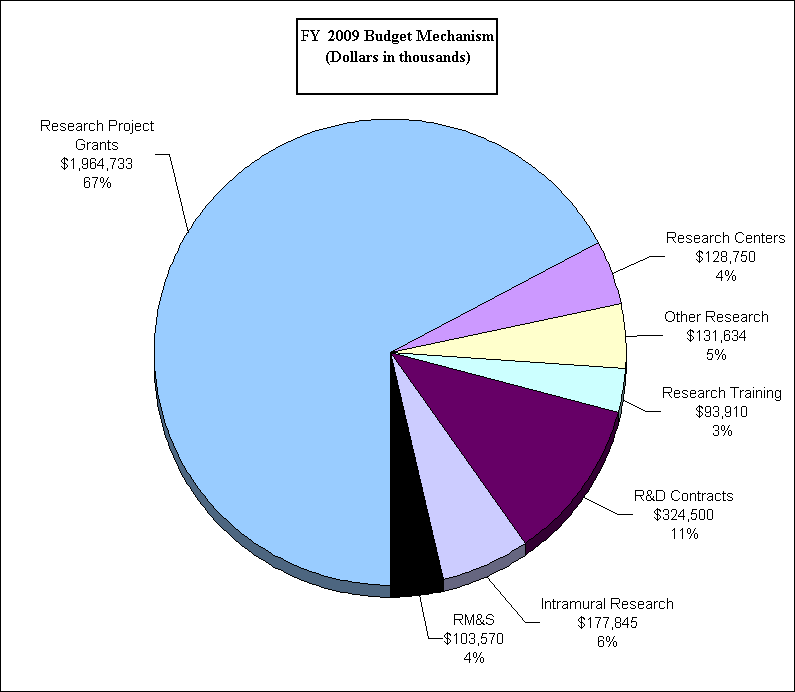
Research Project Grants (-$1.998 million; total $1,964.733 million): The NIH Budget policy for RPGs in FY 2009 is to provide no inflationary increases in noncompeting awards and no increase in average cost for competing RPGs. NHLBI noncompeting grants will increase by $24.393 million in FY 2009. Some of the increased number of grants in the noncompeting mechanism ($6 million) are a result of the Pathways to Independence (K99/R00) program, which will be converted to R00s in FY 2009. NHLBI competing RPGs will decrease by $26.733 million from FY 2008. This decrease will result in the institute funding 54 fewer competing awards. Intramural Research and Research Management Support receive modest increases to help offset the cost of pay and other increases. NHLBI will continue to support new investigators and to maintain an adequate number of competing RPGs.
Research Careers (+/- $0; total $74.834 million): NHLBI will continue to support the Pathway to Independence Program by funding an additional 22 awards in FY 2009. Funds are available as the first round of K99s convert to the R00 mechanism.
Systolic Blood Pressure Intervention Trial (SPRINT) (+$3.070 million): This initiative will support a multicenter randomized trial to determine whether treating systolic blood pressure to a lower goal than is currently recommended can reduce cardiovascular disease.
Medical Education for K-12 Teachers and Students (MKITS II) (+$3.025 million): NHLBI will develop a program, under a contract, that enables experienced PIs to help NHLBI disseminate and evaluate K-12 science education curricula, materials, and educational activities that incorporate best practices learned from previous such programs. These objectives remain unchanged from NHLBI’s original program released in FY 2003.
Integrated Analytical Methods for Large-Scale Multi-dimensional Biomarker, Phenotype, and Genotype Data from the Framingham Biomarkers Project (+$4.0 million): This new project will build upon the Framingham Biomarkers Project by supporting the design of new data integration and analysis methods for extensive heterogeneous biological data and their application to large population studies. Such innovative analytical approaches would seek to model biological mechanisms and disease development processes relevant to the NHLBI mission.
Translating Discoveries in the Behavioral Sciences to Reduce Obesity and Promote Cardiovascular Health (+$5.0 million): This research program would seek to translate findings from basic research on human behavior into effective clinical, community, and population interventions to reduce obesity and promote cardiovascular health. NHLBI anticipates funding 7 awards starting in FY 2009.
| FY 2008 Enacted | $2,922,112,000 |
|---|---|
| FY 2009 estimated budget authority | 2,924,942,000 |
| Net change | 2,830,000 |
2008
Enacted
Base |
Change
from Base |
|||
|---|---|---|---|---|
CHANGES |
FTEs |
Budget Authority |
FTEs |
Budget Authority |
| A. Built-in: | ||||
| 1. Intramural research: | ||||
|
a. Annualization of January 2008 pay increase |
$64,071,000 |
$719,000 |
||
| b. January FY 2009 pay increase | 64,071,000 | 1,394,000 | ||
| c. One less day of pay | 64,071,000 | (245,000) | ||
|
d. Payment for centrally furnished services |
28,853,000 | 433,000 | ||
|
e. Increased cost of laboratory supplies,
materials, and other expenses |
82,210,000 |
1,489,000 |
||
| Subtotal | 3,790,000 | |||
| 2. Research management and support: | ||||
|
a. Annualization of January 2008 pay increase |
$52,421,000 |
$588,000 |
||
| b. January FY 2009 pay increase | 52,421,000 | 1,140,000 | ||
| c. One less day of pay | 52,421,000 | (200,000) | ||
| d. Payment for centrally furnished services | 18,095,000 | 271,000 | ||
|
e. Increased cost of laboratory supplies, materials, and other expenses |
31,474,000 |
562,000 |
||
| Subtotal | 2,361,000 | |||
| Subtotal, Built-in | 6,151,000 | |||
2008
Enacted Base |
Change
from Base |
|||
|---|---|---|---|---|
| CHANGES | No. | Amount | No. | Amount |
| B. Program: | ||||
| 1. Research project grants: | ||||
| a. Noncompeting | 2,801 | $1,448,676,000 | 102 | $24,485,000 |
| b. Competing | 896 | 446,805,000 | (54) | (26,733,000) |
| c. SBIR/STTR | 169 | 71,250,000 | 1 | 250,000 |
| Total | 3,866 | 1,966,731,000 | 49 | (1,998,000) |
| 2. Research centers | 48 | 128,750,000 | 0 | 0 |
| 3. Other research | 669 | 131,634,000 | 0 | 0 |
| 4. Research training | 1,965 | 93,373,000 | 0 | 537,000 |
| 5. Research and development contracts | 207 | 324,500,000 | 0 | 0 |
| Subtotal, extramural | (1,461,000) | |||
FTEs |
Amount |
FTEs |
Amount |
|
| 6. Intramural research | 414 | 175,134,000 | 0 | (1,079,000) |
| 7. Research management and support | 401 | 101,990,000 | 6 | (781,000) |
| 8. Construction | 0 | 0 | ||
| 9. Buildings and Facilities | 0 | 0 | ||
| Subtotal, program | 2,922,112,000 | (3,321,000) | ||
| Total changes | 815 | 6 | 2,830,000 | |

Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
FY 2007 Actual |
FY 2008 Enacted |
FY 2009 Estimate |
Increase
or Decrease |
||||
|---|---|---|---|---|---|---|---|
| FTE | BA | FTE | BA | FTE | BA | FTE | BA |
| 814 | $2,919,180,000 | 815 | $2,922,112,000 | 821 | $2,924,942,000 | 6 | $2,830,000 |
This document provides justification for the Fiscal Year (FY) 2009 activities of the National Heart, Lung, and Blood Institute (NHLBI), including HIV/AIDS activities. Details of the FY 2009 HIV/AIDS activities are in the “Office of AIDS Research (OAR)” Section of the Overview. Details on the Common Fund are located in the Overview, Volume One. Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
The NHLBI provides global leadership for a research and education program to promote prevention and treatment of heart, lung, and blood diseases. The vision is to enhance the health of all individuals and thereby enable them to lead longer and happier lives.
To achieve this vision, NHLBI supports and guides research on the prevention,
causes, diagnosis, and treatment of heart, blood vessel, lung, and blood
diseases. The NHLBI supports individuals in private and public sectors
working in fields related to its mission area, and the education of investigators
working across the spectrum of scientific discovery. The NHLBI creates
and supports a robust, collaborative research infrastructure in partnership
with private and public organizations, including academic institutions,
industry, and government agencies, to address the scientific and educational
needs of the nation. The NHLBI collaborates with individuals, families,
communities, physicians, scientists, health care professionals, professional
societies, patient advocacy groups, and the media to ensure wide dissemination
and maximal use of knowledge to reduce human suffering and benefit individual
and public health.
The pursuit of this vision and mission occurs in a spirit of service that
exemplifies excellence, innovation, integrity, respect, and compassion.
With the extensive involvement of the communities it serves, the NHLBI’s recently completed development of a scientific working plan to guide its activities and initiatives in the near future. Shaping the Future of Research: A Strategic Plan for the National Heart, Lung, and Blood Institute is on the NHLBI Web site at http://apps.nhlbi.nih.gov/strategicplan/.
The plan is structured around three goals that reflect the successive
movement of scientific discovery—from “form to function,”
“function to causes,” and “causes to cures”—and
inform and complement one another. This crosscutting, versus disease-specific,
approach highlights areas where the NHLBI is well positioned to make major
contributions through investigator-initiated research and through programs
that enable and complement investigator-initiated activities.
The plan identifies a number of basic research areas of focus with the
intent of delineating normal and pathological biological mechanisms and
exploiting the emerging understanding of these mechanisms to identify
biomarkers of disease. Such biomarkers—broadly defined as measurable
indicators of genotype, biological or pathological processes, or responses
to therapeutic intervention—will facilitate identification of disease
subtypes and point the way toward new molecular targets for preempting
and preventing disease, as well as for diagnosis and
treatment.
The plan’s clinical and translational research goal emphasizes transmission of knowledge between basic and clinical research so that findings in one arena rapidly inform and stimulate research in the other. More precise methods of risk-stratification and diagnosis are expected to arise from application of new approaches (e.g., noninvasive imaging, biomarkers) from basic science laboratories. A critical challenge will be to develop personalized preventive and therapeutic regimens based on genetic makeup in combination with developmental and environmental exposures. Insights are already emerging, but robust and efficient means of validating both individualized and population-based treatments will be needed to establish an evidence base to guide medical practice.
The plan acknowledges the need to enhance understanding of the processes involved in translating research into practice and to use that understanding to enable improvements in public health and stimulate further scientific discovery. It places particular emphasis on conducting research in primary prevention and identifying interventions that work in the practice communities that will ultimately constitute the targets for translation and education. As well, continued development and evaluation of new approaches to communicate research advances to the public is an important priority for ensuring full and informed participation of individuals in their health care.
The plan is intended to provide the NHLBI with a guide for its research and training programs over the next 5 to 10 years. It presents broad strategies that the NHLBI will employ to facilitate the conduct of research; enhance interdisciplinary work; speed early-stage translation of basic discoveries; ensure cross-fertilization of basic, clinical, and epidemiologic discoveries, and maximize the resultant public health benefit of the information created. Specific measures to implement the plan will now be developed in consultation with the National Heart, Lung, and Blood Advisory Council and the scientific community. Investigator-initiated research has long constituted the largest share of the NHLBI research portfolio, and we fully expect that much of the plan will be realized through continued support of such research. Institute-initiated investments guided by the plan will be directed largely toward programs that either enable or complement investigator-initiated activities.
As the challenges identified in the plan are met and as new ones emerge, the NHLBI will identify and embrace new strategies. The Institute also will continue to look to its Advisory Council and to the larger research community for guidance to ensure that these strategies are updated as needed to reflect the rapidly changing environments of research and public health issues.
Overall Budget Policy: Investigator-initiated research projects and new investigator research and career development remain the Institute’s highest priorities. The NHLBI carefully evaluates investigator-initiated requests to submit grants applications for all large programs. A scientific review is conducted, and the results are presented to the NHLBI Advisory Council to determine level of recommended support, if any. The level of support provided for Institute-initiated projects (e.g. RFAs) is also evaluated. The Institute maintains a balance between solicitations issued to the extramural community in areas that need stimulation and funding made available to support investigator-initiated projects.
Heart and Vascular Diseases: This program seeks to increase knowledge of the causes of heart and vascular diseases and develop effective strategies to diagnose, treat, and prevent them. Diseases and conditions of interest include coronary heart disease, arrhythmias and sudden cardiac death, congenital heart disease, heart failure, hypertension, and peripheral vascular disease.
In fiscal year 2007, the NHLBI initiated a 5-year program to develop and support a Cardiovascular Disease Research Network. Its purpose is to increase scientific knowledge of cardiovascular diseases—including epidemiology, risk and risk factors, prevention, detection and diagnosis, treatment, and prognosis—in the context of participatory, community-based health-care delivery. The Institute awarded 8 cooperative agreements to establish a network for cardiothoracic surgical interventions in cardiovascular medicine. Panels of experts in the fields of lipids and hypertension were convened to assess the needs and opportunities for new clinical trials of cholesterol and blood pressure lowering to prevent cardiovascular disease. The Institute held working groups to identify future research opportunities in two large-scale epidemiological studies—the Multi-ethnic Study of Atherosclerosis and the Coronary Artery Risk Development in Young Adults Study.
Budget Policy: The FY 2009 budget estimate for the Heart and Vascular Diseases program is $1,627,986,000, a decrease of $899,000 or .055% less than the FY 2008 estimate. During the FY 2009 NHLBI plans to continue to support research on the biology of the development of the heart and vascular system and the relationship between cardiovascular physiology and the function of other organs, particularly the lungs, brains, and kidneys. The NHLBI will continue to fund research that uses systems biology approaches and thereby enhances interactions with programs supported by other NIH components. NHBLI also will continue to support work on improving understanding of the characteristics of stem cells in multiple systems and will emphasize translation of fundamental knowledge of stem cell biology into approaches to repair and regenerate damaged tissues and organs.
Population and community-based studies will be supported
to improve the outcomes of resuscitation after myocardial infarction,
to enhance the management of acute coronary syndromes, and to develop
better biomarkers and imaging approaches for identifying pre-clinical
cardiovascular disease and implementing preventive, preemptive
interventions. Research ranging from basic investigations to clinical
studies that address management of heart failure and understanding of
cardiac energetics will be supported.
|
Lung Diseases: This program seeks to understand the causes and progression of lung diseases and sleep disorders and thereby improve their diagnosis, treatment, and prevention. Research areas include asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, critical care and acute lung injury, developmental biology and pediatric pulmonary diseases, immunology and fibrosis, lung cell and vascular biology, and pulmonary complications of AIDS and tuberculosis. The National Center on Sleep Disorders Research is administered as part of the Lung Diseases program.
In fiscal year 2007, the NHLBI initiated a program to foster research on lung stem cell biology and cell-based therapy for lung diseases. It will support collaborations between basic scientists and clinical investigators to advance these promising areas of investigation. In concert with the National Cancer Institute, the NHLBI convened the workshop “Lung Cancer and COPD, Different Outcomes of a Common Etiopathogenetic Pathway?” to identify areas for joint research efforts. Workshops were also held to discuss the future structure and direction of lung diseases clinical research networks, to explore newly identified cellular components of the lung, and to assess the role of circadian timing in the function of cells that make up heart, lung, and blood tissues.
Budget Policy: The FY 2009 budget estimate for the Lung Diseases program is $599,164,000 a decrease of $332,000 or .055% less then the FY 2008 estimate. Plans for FY 2009 continue to emphasize research on lung development, inflammation, injury and repair. Research topics to be investigated include gene therapy for cystic fibrosis, prenatal origins of asthma, and the early indicators of COPD, as detected by biomarkers and imaging. The ultimate goals are to preempt the development of acquired lung diseases and to enhance early detection and thereby prevent or limit disease progression.
The FY 2009 budget estimate for the National Center for Sleep Disorders Research is $50,560,000 the same level as the FY 2008 estimate. The plan for FY 2009 is to continue to support the area of sleep disorders research.
|
Blood Diseases and Resources: This program supports research on the causes, prevention, and treatment of nonmalignant blood diseases, including anemias, sickle cell disease, and thalassemia; premalignant processes such as myelodysplasia and myeloproliferative disorders; abnormalities of hemostasis and thrombosis such as hemophilia; and immune dysfunction. A major program responsibility is to conduct research to ensure the adequacy and safety of the nation's blood supply.
In fiscal year 2007, the NHLBI initiated a program of Pediatric Transfusion Medicine Academic Career Awards. Its goal is to develop curricula designed to attract new investigators into the field of pediatric transfusion medicine. A working group titled “The Interface Between Thrombosis and Inflammation” was convened to achieve a better understanding of how acute and chronic inflammation leads to thrombosis (blood coagulation) and, conversely, how coagulation affects inflammatory activity.
Budget Policy: The FY 2009 budget estimate for the Blood Diseases and Resources program is $416,377,000 a decrease of $230,000 or .055% less than the FY 2008 estimate. During FY 2009, the program plans to continue its support for studies of bone marrow failure, thrombosis, and coagulation disorders, and intrinsic disorders of red cells, white cells, and platelets. Priorities include development of better hematopoietic stem cell therapies and a clearer understanding of the nature of the stem cells that participate in the development of the hematopoietic and immunologic systems. Considerable new knowledge about the interaction between the vascular wall and the coagulation system has expanded the blood program into study of organ damage that arises from thrombosis. Networks for research on hemoglobinopathies (sickle cell disease and the thalassemias) continue to explore approaches for personalizing assessments of disease severity and prognosis and preventing organ damage related to disordered blood flow and iron overload.
The NHLBI will continue to support work to enhance blood safety and ensure
the adequacy of the nation’s blood supply, including studies of
improved component therapies, better procurement and testing, and alternatives
to transfusion. Supportive care with cellular therapies is also a component
of this program. A transfusion medicine network continues innovative work
on approaches to improving the global blood supply, emphasizing enhanced
access, lower costs, and more effective therapies.
|
The Intramural Research program conducts laboratory and clinical research in heart, vascular, lung, blood, and kidney diseases and develops technology related to cardiovascular and pulmonary diseases. The program comprises four centers (Biochemistry and Biophysics, Cell Biology and Physiology, Genetics and Developmental Biology, and Immunology), four branches (Cardiovascular, Hematology, Pulmonary Critical Care Medicine, and Vascular Medicine), and the Cardiothoracic Surgery Research Program.
Budget Policy: The FY 2009 budget estimate for the Intramural Research program is $177,845,000, an increase of $2,711,000 or 1.5% from the FY 2008 estimate. The program plans for FY 2009, along with expected outputs, are as follows. Increases for salaries and related costs are covered in the budget request. The budget provides support for new programs, which include (a) expansion of the newly formed Pulmonary and Vascular Medicine Branch to include two new tenure track scientists, one working on sickle cell disease, the other in lipid metabolism therapeutics, (b) recruitment of one tenure track scientists in the basic sciences in physical biology, (c) recruitment of one tenure track scientist in the general clinical sciences, (d) using technology developed within the DIR to establish a epigenetic screening center for the human genome, (e) initiation of a state of the art computer tomography system for the study of heart disease, (f) continued expansion of the post-translational modification detection capabilities in the proteomic core, (g) establish a state of the heart cardiovascular phenotyping center for the mouse, (h) further develop systems biology platforms for the analysis of proteomic and genomic data, (i) further develop the in vivo optical microscopy program using adaptive optics, motion compensation and novel detection systems to reach sub-micron resolution in intact animal models, (j) expand the robotic and catheter fabrication capabilities within the DIR to support MRI guided therapeutic approaches applications in man.
This activity provides administrative management and scientific direction in the review, award, and monitoring of research grants, training awards and research and development contracts and in the overall planning, coordination, and evaluation of the Institute’s programs.
In fiscal year 2008, the Division of Extramural Research Activities administered the review, processing, award, and scientific performance appraisal of approximately 5,000 research grants, 750 training awards, and 600 contracts. The Division for the Application of Research Discoveries continued its public and professional education activities in cardiovascular diseases and asthma and developed a new educational campaign, “COPD: Learn More, Breathe Better.”
Budget Policy: The FY 2009 budget estimate for Research Management and Support is $103,570,000, an increase of $1,580,000 or 1.5% over the FY 2008 estimate. The program plans for FY 2009, along with expected outputs, are as follows. Increases for salaries and related costs are covered in the budget request, but decreases are planned for operating expenses such as maintenance contracts and information technology costs. The NHLBI Division for the Application of Research Discoveries will continue with the planning and implementation of knowledge networks to support rapid sharing of knowledge and experience between researchers and practitioners. Establishment of the initial Cardiovascular Knowledge Network will be followed closely by the establishment of the Pulmonary Knowledge Network to address asthma and COPD.
In regard to its lifespan approach to addressing prevention and treatment of cardiovascular disease, the Division will conduct activities to follow the issuance of the first-ever integrated guidelines for reduction of cardiovascular risk factors in children ages 2 to 22. FY 09 activities will include implementation of educational efforts directed at health care professionals as well as a consumer-oriented education and outreach campaign to facilitate and enhance participatory medicine.
In the asthma area, the Division will design and implement similar efforts based on the asthma management and treatment guidelines issued in late 2007. Education activities will be developed for children, adolescents, and adults as well as for health care professionals in the two related campaigns.
|
OBJECT CLASSES |
FY 2008 Enacted |
FY 2009 Estimate |
Increase
or Decrease |
|---|---|---|---|
| Personnel Compensation: | |||
| Full-time permanent (11.1) | $66,869,000 | $70,544,000 | $3,675,000 |
| Other than full-time permanent (11.3) | 13,758,000 | 14,391,000 | 633,000 |
| Other personnel compensation (11.5) | 3,068,000 | 3,209,000 | 141,000 |
| Military personnel (11.7) | 1,301,000 | 1,360,000 | 59,000 |
| Special personnel services payments (11.8) | 9,038,000 | 9,462,000 | 424,000 |
| Total Personnel Compensation (11.9) | 94,034,000 | 98,966,000 | 4,932,000 |
| Civilian personnel benefits (12.1) | 21,603,000 | 22,797,000 | 1,194,000 |
| Military personnel benefits (12.2) | 856,000 | 896,000 | 40,000 |
| Benefits to former personnel (13.0) | 0 | 0 | 0 |
| Subtotal, Pay Costs | 116,493,000 | 122,659,000 | 6,166,000 |
| Travel (21.0) | 3,197,000 | 3,070,000 | (127,000) |
| Transportation of things (22.0) | 295,000 | 280,000 | (15,000) |
| Rental payments to others (23.2) | 210,000 | 200,000 | (10,000) |
| Communications,
utilities and miscellaneous charges (23.3) |
1,413,000 |
1,375,000 |
(38,000) |
| Printing and reproduction (24.0) | 687,000 | 660,000 | (27,000) |
| Other Contractual Services: | |||
| Advisory and assistance services (25.1) | 1,779,000 | 1,550,000 | (229,000) |
| Other services (25.2) | 14,832,000 | 14,200,000 | (632,000) |
| Purchases from government accounts (25.3) | 22,060,000 | 14,908,000 | (7,152,000) |
| Operation and maintenance of facilities (25.4) | 3,550,000 | 3,400,000 | (150,000) |
| Operation and maintenance of equipment (25.7) | 6,960,000 | 6,650,000 | (310,000) |
| Subsistence and support of persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | 49,181,000 | 40,708,000 | (8,473,000) |
| Supplies and materials (26.0) | 16,206,000 | 15,560,000 | (646,000) |
| Subtotal, Non-Pay Costs | 71,189,000 | 61,853,000 | (9,336,000) |
| Total, Administrative Costs | 187,682,000 | 184,512,000 | (3,170,000) |
| Fiscal Year |
Budget Estimate to Congress |
House Allowance |
Senate Allowance |
Appropriation(1) |
|---|---|---|---|---|
| 2000 | 1,759,806,000 (2) | 1,937,404,000 | 2,001,185,000 | 2,040,291,000 |
| Rescission | (10,867,000) | |||
| 2001 | 2,069,582,000 (2) | 2,321,320,000 | 2,328,102,000 | 2,299,100,000 |
| Rescission | (875,000) | |||
| 2002 | 2,567,429,000 | 2,547,675,000 | 2,618,966,000 | 2,576,125,000 |
| Rescission | (3,063,000) | |||
| 2003 | 2,778,728,000 | 2,791,411,000 | 2,820,011,000 | 2,812,011,000 |
| Rescission | (18,278,000) | |||
| 2004 | 2,867,995,000 | 2,867,995,000 | 2,897,595,000 | 2,897,145,000 |
| Rescission | (18,454,000) | |||
| 2005 | 2,963,953,000 | 2,963,953,000 | 2,985,900,000 | 2,965,453,000 |
| Rescission | (24,252,000) | |||
| 2006 | 2,951,270,000 | 2,951,270,000 | 3,023,381,000 | 2,951,270,000 |
| Rescission | (29,513,000) | |||
| 2007 | 2,918,808,000 | 2,901,012,000 | 2,924,299,000 | 2,918,808,000 |
| 2008 | 2,894,341,000 | 2,965,775,000 | 2,992,197,000 | 2,974,900,000 |
| Rescission | (51,972,000) | |||
| 2009 | 2,924,942,000 |
OFFICE/DIVISION |
FY 2007 Actual |
FY 2008 Enacted |
FY 2009 Estimate |
|---|---|---|---|
| Office of the Director | 15 | 15 | 15 |
| Office of Biostatistics Research | 12 | 12 | 12 |
| Office of Science and Technology | 18 | 18 | 18 |
| Office of Administrative Management | 69 | 69 | 69 |
| Office of Research Training and Minority Health | 4 | 4 | 4 |
| Office of Clinical Research | 5 | 5 | 5 |
| Division of Cardiovascular Diseases | 57 | 57 | 59 |
| Division of Prevention and Population Sciences | 43 | 43 | 46 |
| Center for Population Studies | 4 | 4 | 4 |
| Center for Biomedical Informatics | 17 | 17 | 18 |
| Division of Lung Diseases | 22 | 22 | 22 |
| Division of Blood Diseases and Resources | 23 | 23 | 23 |
| Division of Intramural Research | 386 | 386 | 386 |
| Division of Extramural Research Activities | 104 | 105 | 105 |
| Division Application Research Discoveries | 35 | 35 | 35 |
| Total | 814 | 815 | 821 |
FISCAL
YEAR |
Average
GM/GS Grade |
|---|---|
2005 |
12.2 |
2006 |
13.0 |
2007 |
12.1 |
2008 |
12.2 |
2009 |
12.2 |
GRADE |
FY 2007 Actual |
FY 2008 Enacted |
FY 2009 Estimate |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 166,102 | 173,560 | 178,767 |
| GM/GS-15 | 97 | 97 | 97 |
| GM/GS-14 | 113 | 113 | 113 |
| GM/GS-13 | 138 | 139 | 141 |
| GS-12 | 94 | 96 | 97 |
| GS-11 | 34 | 35 | 36 |
| GS-10 | 2 | 2 | 2 |
| GS-9 | 55 | 56 | 57 |
| GS-8 | 32 | 33 | 33 |
| GS-7 | 17 | 17 | 18 |
| GS-6 | 11 | 11 | 11 |
| GS-5 | 7 | 7 | 7 |
| GS-4 | 5 | 5 | 5 |
| GS-3 | 1 | 1 | 1 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 606 | 612 | 618 |
| Grades established
by Act of July 1, 1944 (42 U.S.C. 207): |
|||
| Assistant Surgeon General | 1 | 1 | 1 |
| Director Grade | 7 | 7 | 7 |
| Senior Grade | 2 | 2 | 2 |
| Full Grade | 1 | 1 | 1 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 11 | 11 | 11 |
| Ungraded | 252 | 255 | 258 |
| Total permanent positions | 720 | 727 | 734 |
| Total positions, end of year | 870 | 879 | 888 |
| Total full-time equivalent (FTE) | |||
| employment, end of year | 814 | 815 | 821 |
| Average ES salary | 166,102 | 173,560 | 178,767 |
| Average GM/GS grade | 12.1 | 12.2 | 12.2 |
| Average GM/GS salary | 89,529 | 93,549 | 96,355 |
FY 2009 |
|||
|---|---|---|---|
| Grade | Number | Annual Salary |
|
| Health Scientist Administrator | 13 | 2 | $94,000 |
| Project Manager | 13 | 1 | $94,000 |
| Procurement Analyst | 14 | 1 | $111,000 |
| Clinical Trial Specialist | 13 | 2 | $94,000 |
| Total Requested | 6 | ||
|