Sawyer, child in Fabry disease study
Why Do Research In Children?
Medicines, devices and treatments are often not tested in children.
At nearly half of medical visits, children are given a medicine and 70% of those medicines have only been tested in adults.
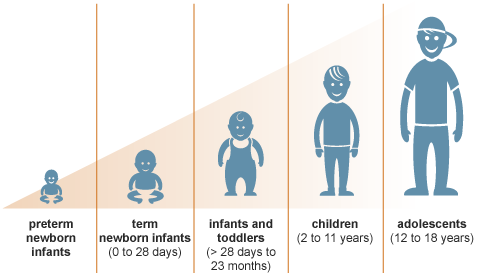
The simple truth is...children are not little adults.
But without research in children themselves, we have no choice but to treat them that way.
Doctors and nurses often give medicines to children even though they have not been studied and approved by the Food and Drug Administration (FDA) for use in children. This is known as "off-label" use. Most of the time, this works well but when the adult dose is adjusted to the weight of a child, there is a chance that the dose used could be ineffective or even harmful.
While it may sound like "guesswork," it's really been more of a 'hand-me-down' approach. Without research in children though, it's all we have. We need to think about how a child's brain and body are developing...as well as the way that medicines and other treatments are handled in a child's body over time.
Because?
Jackie, mother of child in Fabry disease study
Clinical studies are important
- Understand the differences in children as they grow and develop.
- Identify the best dose of medicines to prevent harmful effects or under-treatment.
- Produce chewables, liquids or tablets that are easier for children to take.
- Find treatments for problems that occur only in children, like prematurity.
- Find treatments for certain diseases or conditions that occur in both children and adults but which act differently in children and adults, like arthritis or heart disease.
- Understand how medicines are used in and filtered out of the body in children of all ages.
- Find treatments for new or existing diseases to improve the health of children in the future, like vaccine studies that were done years ago help children stay healthier today.
- Treat our children like children, rather than little adults.
Nicole, mother of child in heart defect study
 Previous
Previous
