Contact Us
- 800–CDC–INFO
- (800-232-4636)
- 888–232–6348 (TTY)
- cdcinfo@cdc.gov
Flu Vaccine Effectiveness: Questions and Answers for Health Professionals
On this page
- How is influenza vaccine effectiveness measured?
- Why does influenza vaccine effectiveness vary?
- How effective is the inactivated influenza vaccine (IIV)?
- How effective is the live attenuated influenza vaccine (LAIV)?
- How does vaccine efficacy/effectiveness (VE) compare between live attenuated vaccine and inactivated vaccine?
- What information is necessary for yearly surveillance of vaccine effectiveness?
- What types of vaccine effectiveness studies are being conducted by CDC now?
How is influenza vaccine effectiveness measured?
Vaccine efficacy and effectiveness studies use various endpoints or outcomes, which influence how we interpret the results. These endpoints may include the prevention of medically attended acute respiratory illness (MAARI), prevention of laboratory-confirmed influenza virus illness or hospitalization, prevention of influenza-like illness (ILI, such as illness with fever and cough or sore throat), or influenza-associated hospitalizations or deaths. Studies that use outcomes such as an influenza laboratory-confirmed outcome provide the most specific estimates of the impact of the vaccine in preventing influenza. The more non-specific the outcome being measured (e.g., all pneumonia hospitalizations or influenza-like illness that include many illnesses not caused by the influenza virus), the lower the estimates of vaccine effectiveness. For example, a study by Bridges et al. (JAMA 2000) among healthy adults found that the inactivated influenza was 86% effective against laboratory-confirmed influenza, but only 10% effectiveness against all respiratory illnesses in the same population and year.
Why does influenza vaccine effectiveness vary?
The effectiveness of inactivated influenza vaccine depends primarily on the age and immunocompetence of the vaccine recipient, and the degree of similarity between the viruses in the vaccine and those in circulation. In years when the vaccine strains are not well matched to circulating strains, vaccine effectiveness is generally lower. The vaccine may also be lower among persons with chronic medical conditions and among the elderly, as compared to healthy young adults and children. In addition, estimates of vaccine effectiveness vary, based on the specificity of the outcome that is being measured in the study.
It is also important to keep in mind that measurement of vaccine effectiveness against a non-laboratory confirmed outcome is at least partially determined by how much of that outcome is actually caused by influenza viruses, as compared to other pathogens. For example, one non-laboratory confirmed outcome that is often used is ILI. The proportion of ILI’s caused by influenza versus other pathogens can vary by year, or even within a year.
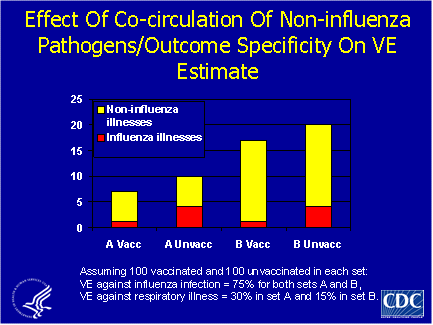
As illustrated in the theoretical example graphed below, there is a relationship between our estimates of vaccine efficacy and the proportion of all ILI’s caused by influenza versus other pathogens. In this example, the vaccine was 75% effective against laboratory-confirmed influenza, but was 30% effective against ILI when influenza caused 40% of ILI’s in the unvaccinated group. The vaccine would be only 15% effective, however, if influenza caused only 20% of ILI’s. This is an important relationship to keep in mind, since there can be wide variation in the percentage of ILIs caused by influenza.
How effective is the inactivated influenza vaccine (IIV)?
Overall
Overall, in years when the vaccine and circulating viruses are well-matched, influenza vaccines can be expected to reduce laboratory-confirmed influenza by approximately 70% to 90% in healthy adults <65 years of age. Several studies have also found reductions in febrile illness, influenza-related work absenteeism, antibiotic use, and doctor visits.
In years when the vaccine strains are not well matched to circulating strains, vaccine effectiveness can be variably reduced. For example, in a study among persons 50-64 years during the 2003-04 season, when the vaccine strains were not optimally matched, inactivated influenza vaccine effectiveness against laboratory-confirmed influenza was 60% among persons without high-risk conditions, and 48% among those with high risk conditions, but it was 90% against laboratory-confirmed influenza hospitalization (Herrera, et al Vaccine 2006). A study in children during the same year found vaccine effectiveness of about 50% against medically diagnosed influenza and pneumonia without laboratory confirmation (Ritzwoller, Pediatrics 2005). However, in some years when vaccine and circulating strains were not well-matched, no vaccine effectiveness can be demonstrated in some studies, even in healthy adults (Bridges, JAMA 2000). It is not possible in advance of the influenza season to predict how well the vaccine and circulating strains will be matched, and how that match may affect the degree of vaccine effectiveness.
Immunocompromised
The immune competence of the person being vaccinated can also affect vaccine effectiveness. For example, the vaccine may be only 30%-40% effective against influenza-related respiratory illness among nursing home residents. However, even in this group of frail elderly, the vaccine still provides substantial protection against more severe outcomes, such as influenza-related hospitalization (effectiveness of 50-60%) and deaths (80%). The presence of chronic medical conditions may also affect vaccine effectiveness. In a study of persons 50-64 years of age, the vaccine was 60% effective among otherwise healthy adults 50-64 years of age, but only 48% effective among those with high-risk medical conditions (Herrera, et al Vaccine 2006).
Adults 65 years or older
Among elderly persons not living in nursing homes or similar long-term care facilities, influenza vaccine has been reported to be 30%-70% effective in preventing hospitalization for pneumonia and influenza. Among older persons who reside in nursing homes, influenza vaccine is most effective in preventing severe illness, secondary complications, and deaths. Among this population, the vaccine has been reported to be 50%-60% effective in preventing influenza-related hospitalization or pneumonia, and 80% effective in preventing influenza-related death, although the effectiveness in preventing illness from influenza often ranges from 30% to 40%. In years when the vaccine is not well- matched to circulating influenza strains, vaccine effectiveness (VE) is often lower.
For nursing homes, vaccination rates of 80% or more among residents can reduce the risk of influenza outbreaks in the facility. In addition, other studies suggest that the vaccination of the health care workers in nursing homes, as well as the residents, is important to prevent influenza outbreaks (Potter et al., J Infect Dis 1997;175:1—6. Carman et al., Lancet 2000;355(9198):93—7. Shugarman et al., J Am Med Dir Assoc. 2006 Nov;7(9):562-7).
Children
A 4-year randomized, placebo-controlled trial of children aged 1-15 years found vaccine effectiveness ranging from 77% to 91%, following only one dose of vaccine given to previously unvaccinated children (Neuzil, Pediatric Infectious Diseases Journal, 2001).
Another 2-year study of children aged 6-24 months found that the vaccine was 66% effective against laboratory-confirmed influenza in year 1 of the study. Only children who were fully vaccinated (i.e. had either 2 doses if not previously vaccinated, or 1 dose if previously vaccinated) versus unvaccinated children were included in the analysis. In the other year, few cases of influenza occurred, making it difficult to assess the vaccine’s effectiveness.
A study of influenza vaccine effectiveness among >5,000 children aged 6-23 months found vaccine effectiveness of 49% against clinically diagnosed pneumonia or influenza among fully vaccinated children (Ritzwoller, Pediatrics 2005).
All of these studies together suggest substantial benefit from influenza vaccination of children.
How does the number of doses of vaccine that a child receives affect vaccine effectiveness?
Children <9 years of age who have not been vaccinated previously are recommended to receive 2 doses the first year they get vaccinated. In subsequent years, they need only 1 dose. This is because many children <9 years of age have not been infected with influenza viruses previously, and a booster dose is needed in children previously not exposed to influenza infections or vaccine in order for them to have a good immune response.
For the inactivated vaccine, no VE has been demonstrated for children who needed 2 doses but who received only one. (Ritzwoller et al. Pediatrics 2003; Allison MA, et al. J Pediatrics 2006). A study of live attenuated vaccine by Belshse et al of children <5 years, however, did show VE among children administered only 1 dose. However, the live vaccine is not licensed for use in children <5 years of age.
Chronic medical conditions
A limited number of influenza vaccine studies have been conducted in groups with underlying medical conditions. In a study of adults aged 50—64 years with laboratory-confirmed influenza during the 2003-04 season, when vaccine and circulating viruses were not well-matched, vaccine effectiveness was estimated to be 48% among those with one or more high-risk conditions. A study of high-risk adults aged 18-64 found vaccine effectiveness against influenza-related hospitalization and deaths of 87% and 78%, respectively (Hak, 2005). A study of diabetic persons estimated that influenza vaccine reduced influenza, pneumonia, or diabetes-related hospitalizations by 79% (95% CI: 19% to 95%) during two influenza seasons (Colquhoun, 1997). However, the control group had a significantly lower proportion of insulin-dependent diabetics, which could have inflated the vaccine effectiveness estimates.
Overall, vaccine efficacy and effectiveness estimates among persons with high-risk conditions are somewhat lower compared to similar age groups of persons without high-risk conditions. However, the risk of influenza-related complications among this group is much higher, so vaccination provides substantial benefit, even given the lower effectiveness.
How effective is the live attenuated influenza vaccine (LAIV)?
(As of 2007, this vaccine is licensed only for healthy, non-pregnant persons between 5 and 49 years.)
Healthy Children
A randomized, double-blind, placebo-controlled trial among 1,602 healthy children initially aged 15--71 months assessed the efficacy of trivalent LAIV against culture-confirmed influenza during two seasons (Belshe et al., N Engl J Med 1998;338:1405—12. Belshe et al, J Pediatr 2000;136:168--75). In season one, when vaccine and circulating virus strains were well-matched, efficacy in preventing confirmed illness from influenza was 93% for participants who received 2 doses of LAIV. In season two, when the A (H3N2) component was not well-matched between vaccine and circulating virus strains, efficacy was 86% overall.
Other non-laboratory confirmed, less specific outcome results included a 21% reduction in all febrile illnesses, 27% reduction in febrile otitis media, and a 28% reduction in otitis media with concomitant antibiotic use. A review of LAIV effectiveness in children aged 18 months—18 years found effectiveness against medically attended acute respiratory illness (MAARI), a non-laboratory confirmed outcome, of 18%, but greater estimated efficacy against laboratory confirmed influenza-- 92% against influenza A (H1N1), and 66% against an influenza B drift variant (Halloran et al., Am J Epidemiol 2003;158:305--11).
Healthy Adults
A randomized, double-blind, placebo-controlled trial among 4,561 healthy working adults aged 18--64 years assessed multiple endpoints, including reductions in self-reported respiratory tract illness without laboratory confirmation, absenteeism, healthcare visits, and medication use during peak and total influenza outbreak periods (Nichol et al., JAMA 1999;282:137--44). The study was conducted during the 1997--98 influenza season, when the vaccine and circulating A (H3N2) strains were not well-matched. Vaccination was associated with reductions in severe febrile illnesses of 19%, and febrile upper respiratory tract illnesses of 24%.
Vaccination was also associated with fewer days of illness, fewer days of work lost, fewer days with healthcare provider visits, and reduced use of prescription antibiotics and over-the-counter medications. Among a subset of 3,637 healthy adults aged 18--49 years, LAIV recipients (n = 2,411) had 26% fewer febrile upper-respiratory illness episodes; 27% fewer lost work days as a result of febrile upper respiratory illness; and 18%--37% fewer days of healthcare provider visits caused by febrile illness, compared with placebo recipients (n = 1,226). Days of antibiotic use were reduced by 41%--45% in this age subset.
A randomized, double-blind, placebo-controlled challenge study among 92 healthy adults (LAIV, n = 29; placebo, n = 31; inactivated influenza vaccine, n = 32) aged 18--41 years assessed the efficacy of both LAIV and inactivated vaccine (Treanor et al., Vaccine 1999;18:899--906.). The overall efficacy of LAIV and inactivated influenza vaccine in preventing laboratory-documented influenza from all three influenza strains combined was 85% and 71%, respectively. This was on the basis of experimental challenge by viruses to which study participants were susceptible before vaccination. The difference in efficacy between the two vaccines was not statistically significant.
How does vaccine efficacy/effectiveness (VE) compare between live attenuated vaccine and inactivated vaccine?
Few studies directly comparing LAIV and IIV have been done, and results appear to differ for adults versus children. A randomized, placebo-controlled trial among 876 healthy adults 18-46 years old found overall VE of 75% for IIV, which was statistically significant, and 48% VE for LAIV, which was not statistically significant in a year with a drifted strain (Ohmit NEJM 2006). However, a small randomized, double-blind, placebo-controlled challenge study among 92 healthy adults (LAIV, n = 29; placebo, n = 31; inactivated influenza vaccine, n = 32) aged 18-41 years found overall efficacy of LAIV and inactivated influenza vaccine in preventing laboratory-documented influenza of 85% and 71%, respectively (Treanor et al., Vaccine 1999;18:899--906.). The difference in efficacy between the two vaccines was not statistically significant.
However, in 3 studies among children where inactivated vaccine was compared with a refrigerator-stable experimental form of the LAIV, referred to as CAIV-T, which is not yet licensed in the U.S., the LAIV vaccine was overall of greater benefit. However, none of the studies included a placebo group, so overall effectiveness could not be assessed. One study included 2187 children aged 6-71 months who had recurrent respiratory tract infections (Ashkenazi et al. Pediatric Infect Dis J 2006) and found overall influenza rates of 2.3% among CAIV-T recipients, and 4.8% for TIV, for a 52.7% decrease. In a randomized study of 2229 children 6-17 years with asthma, 4.1% of CAIV-T recipients and 6.2% of TIV recipients developed influenza, for a reduction of 34.7% for CAIV-T over TIV (Fleming DM, et al. Pediatric Infect Dis J 2006). Publication of the results of a third study among 7852 children 6-59 months is pending.
What information is necessary for yearly surveillance of vaccine effectiveness?
Ideally, influenza vaccine effectiveness (VE) would be assessed on a yearly basis, using a consistent methodology and population. This would allow for comparison of clinical VE outcomes with laboratory data on the relatedness of influenza vaccine viruses with those contained in the vaccine. Use of a laboratory-confirmed outcome to assess VE is very important to provide the most specific results of the benefit of the vaccine, and to limit the impact of the co-circulation of non-influenza respiratory pathogens on the influenza VE estimate. Ideal populations for assessment would include different analyses of pediatric populations, healthy adults, and older adults. Because a proportion of older adults have co-morbidities and may be differentially motivated to seek medical care for influenza-related symptoms, analyses and interpretation of influenza VE in this population are difficult. Thus, this should not be the only population assessed for influenza VE.
What types of vaccine effectiveness studies are being conducted by CDC now?
Map: Emerging Infections Program and New Vaccine Surveillance Network Participating Counties, 2005-2006
Because of the changing nature of influenza viruses and the vaccine, annual assessment of the effectiveness of influenza vaccines is important. Vaccine effectiveness studies can look at various target populations (e.g., children, the elderly), and determine how well the vaccine prevents against different outcomes (e.g., hospitalization, illness, death, and laboratory-confirmed infection). CDC is currently conducting studies that have only a laboratory-confirmed outcome. The findings of these studies are then used to enhance vaccine recommendations or support the need for new methods of vaccine production.
In 2004, a 3-year CDC-funded pilot study with the Marshfield Clinic Research Foundation was initiated to develop an assessment system of the vaccine’s effectiveness across all groups for whom influenza vaccine is targeted. This study is in its final year.
Through CDC’s Emerging Infections Program Network and the New Vaccine Surveillance Network, other vaccine effectiveness studies are being conducted during the 2006-07 influenza season among children 6-59 months of age. These studies will assess the effectiveness of the vaccine in preventing laboratory-confirmed influenza hospitalizations.
Influenza Vaccine Effectiveness Studies Currently Being Conducted by CDC
| Study Design | Cohort and case-control |
|---|---|
| Seasons | 2004-05, 2005-06, 2006-07 |
| Setting | Clinic population in north-central WI |
| Age | All ACIP-recommended groups |
| Cases | Patients with acute respiratory illness (ARI) symptoms and influenza positive by culture or RT-PCR |
| Cohort Controls | Cohort of adults and children for whom ACIP recommended annual influenza vaccination. Age-matched persons with ARI symptoms in same healthcare system. |
| Vaccination data | Obtained from regional electronic vaccine registry and patient report |
| Source of other data | Electronic medical record and interview of adult or parent/guardian |
| Other patient characteristics included in analyses | Age, gender, race, high- risk condition, use of health care |
| Study Design | Case-control |
|---|---|
| Seasons | 2005-06, 2006-07 |
| Setting | Hospitals in 8 (05-06) and 9 (06-07) states |
| Age | 6-23 mo (05-06), 6-59 mo (06-07) |
| Cases | Children hospitalized with laboratory confirmed influenza test. Testing done as ordered by clinicians. |
| Controls | Age- and zip-code matched children not hospitalized with influenza |
| Vaccination data | Obtained from health care providers and parent report |
| Source of other data | Medical chart review, interview of parent/guardian |
| Other patient characteristics included in analyses | Age, gender, race, insurance status, high- risk conditions, socioeconomic status |
| Study Design | Case-control |
|---|---|
| Seasons | 2003-04, 2004-05, 2005-06, 2006-07 |
| Setting | Hospitals, Emergency Departments, and outpatient clinics in 3 counties (TN, NY, OH) |
| Age | 6-59 months |
| Cases | Children prospectively enrolled with fever or ARI who test positive for influenza by culture or RT-PCR |
| Controls | Children prospectively enrolled with fever or ARI who test negative for influenza by culture and RT-PCR |
| Vaccination data | Obtained from health care providers |
| Source of other data | Medical chart review, interview of parent/guardian |
| Other patient characteristics included in analyses | Date enrolled, age, gender, race, insurance status, high-risk conditions, socioeconomic status and other risk factors. |
- Page last updated January 11, 2006
- Content Source: Coordinating Center for Infectious Diseases (CCID)
- National Center for Immunization and Respiratory Diseases (NCIRD)

