Diagnosis
Spotlight: Standard DFA Protocol For Rabies (PDF – 2.3 MB)

Among the findings of the National Working Group on Rabies Prevention and Control was the need for a minimum national standard for the laboratory diagnosis of rabies. In response to this recommendation, a committee was formed from representatives of national and state public health laboratories to evaluate procedures employed by rabies diagnostic... more » (PDF – 2.3 MB)
Rabies diagnosis in animals
The direct fluorescent antibody test (dFA) is the test most frequently used to diagnose rabies. This test requires brain tissue from animals suspected of being rabid. The test can only be performed post-mortem (after the animal is dead).
Rabies diagnosis in humans
Several tests are necessary to diagnose rabies ante-mortem (before death) in humans; no single test is sufficient. Tests are performed on samples of saliva, serum, spinal fluid, and skin biopsies of hair follicles at the nape of the neck. Saliva can be tested by virus isolation or reverse transcription followed by polymerase chain reaction (RT-PCR). Serum and spinal fluid are tested for antibodies to rabies virus. Skin biopsy specimens are examined for rabies antigen in the cutaneous nerves at the base of hair follicles.
The importance of routine rabies tests
Rapid and accurate laboratory diagnosis of rabies in humans and other animals are essential for timely administration of postexposure prophylaxis. Within a few hours, a diagnostic laboratory can determine whether or not an animal is rabid and inform the responsible medical personnel. The laboratory results may save a patient from unnecessary physical and psychological trauma, and financial burdens, if the animal is not rabid.
In addition, laboratory identification of positive rabies cases may aid in defining current epidemiologic patterns of disease and provide appropriate information for the development of rabies control programs.
Essential characteristics for routine rabies test
The nature of rabies disease dictates that laboratory tests be standardized, rapid, sensitive, specific, economical, and reliable.
Laboratory tests for rabies
The standard test for rabies testing is dFA. This test has been thoroughly evaluated for more than 40 years, and is recognized as the most rapid and reliable of all the tests available for routine use. All rabies laboratories in the United States perform this test (post-mortem) on animals suspected of having rabies.
Other tests for diagnosis and research, such as electron microscopy (EM), histologic examination, immunohistochemistry (IHC), RT-PCR, and isolation in cell culture are useful tools for studying the virus structure, histopathology, molecular typing, and virulence of rabies viruses.
Direct fluorescent antibody test (dFA)
The dFA test is based on the observation that animals infected by rabies virus have rabies virus proteins (antigen) present in their tissues. Because rabies is present in nervous tissue (and not blood like many other viruses), the ideal tissue to test for rabies antigen is brain. The most important part of a dFA test is flouresecently-labelled anti-rabies antibody. When labelled antibody is incubated with rabies-suspect brain tissue, it will bind to rabies antigen. Unbound antibody can be washed away and areas where antigen is present can be visualized as fluorescent-apple-green areas using a fluorescence microscope. If rabies virus is absent there will be no staining.
Antigen detection by dFA
The rabies antibody used for the dFA test is primarily directed against the nucleoprotein (antigen) of the virus (see The Virus section on viral structure). Rabies virus replicates in the cytoplasm of cells, and infected cells may contain large round or oval inclusions containing collections of nucleoprotein (N) or smaller collections of antigen that appear as dust-like fluorescent particles if stained by the dFA procedure.
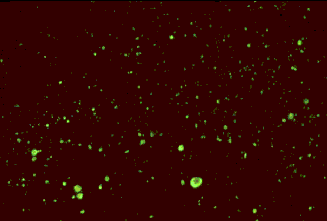
Positive dFA

Negative dFA
General histopathology
Histologic examination of biopsy or autopsy tissues is occasionally useful in diagnosing unsuspected cases of rabies that have not been tested by routine methods. When brain tissue from rabies virus-infected animals are stained with a histologic stain, such as hematoxylin and eosin, evidence of encephalomyelitis may be recognized by a trained microscopist. This method is nonspecific and not considered diagnostic for rabies.
Before current diagnostic methods were available, rabies diagnosis was made using this method and the clinical case history. In fact, most of the significant histopathologic features (changes in tissue caused by disease) of rabies infection were described in the last quarter of the 19th century. After Louis Pasteur's successful experiments with rabies vaccination, scientists were motivated to identify the pathologic lesions of rabies virus.
Histopathologic evidence of rabies encephalomyelitis (inflammation) in brain tissue and meninges includes the following:
- Mononuclear infiltration
- Perivascular cuffing of lymphocytes or polymorphonuclear cells
- Lymphocytic foci
- Babes nodules consisting of glial cells
- Negri bodies
Perivascular cuffing or inflammation around a blood vessel. Perivascular inflammatory cell infiltrates in hematoxylin & eosin stained brain tissue.
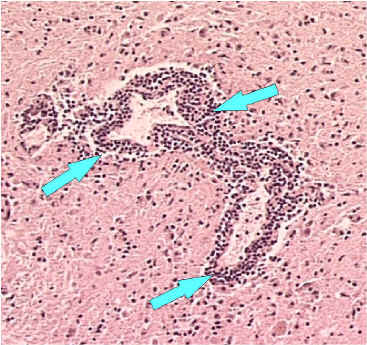
100x Magnification
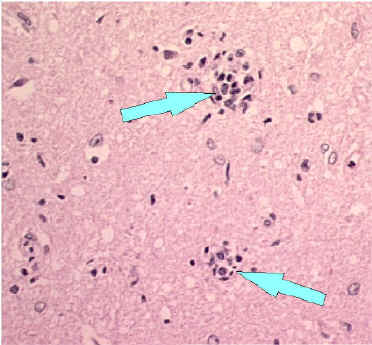
Babes Nodules
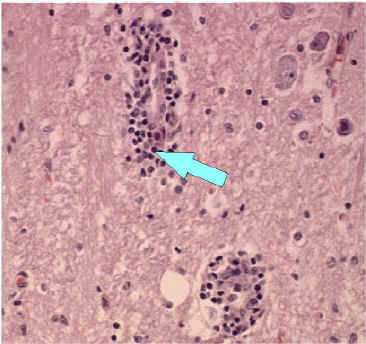
200x Magnification
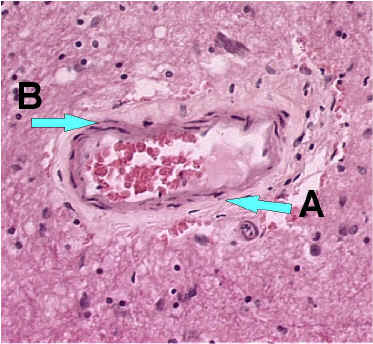
Blood vessel without inflammatory cells (200x magnification)
A. Red blood cells
B. Squamous epithelial cells
Negri bodies
In 1903, most of the histopathologic signs of rabies were recognized, but rabies inclusions had not yet been detected. At this time, Dr. Adelchi Negri reported the identification of what he believed to be the etiologic agent of rabies, the Negri body. In his report, he described Negri bodies as round or oval inclusions within the cytoplasm of nerve cells of animals infected with rabies. Negri bodies may vary in size from 0.25 to 27 µm. They are found most frequently in the pyramidal cells of Ammon's horn, and the Purkinje cells of the cerebellum. They are also found in the cells of the medulla and various other ganglia. Negri bodies can also be found in the neurons of the salivary glands, tongue, or other organs. Staining with Mann's, giemsa, or Sellers stains can permit differentiation of rabies inclusions from other intracellular inclusions. With these stains, Negri bodies appear magenta in color and have small (0.2 µm to 0.5 µm), dark-blue interior basophilic granules.
The presence of Negri bodies is variable. Histologic staining for Negri bodies is neither as sensitive nor as specific as other tests. Some experimentally-infected cases of rabies display Negri bodies in brain tissue; others do not. Histologic examination of tissues from clinically rabid animals show Negri bodies in about 50% of the samples; in contrast, the dFA test shows rabies antigen in nearly 100% of the samples. In other cases, non-rabid tissues have shown inclusions indistinquishable from Negri bodies. Because of these problems, the presence of Negri bodies should not be considered diagnostic for rabies.
H & E-strained tissue
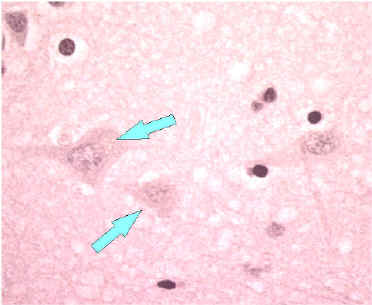
Neuron without Negri bodies
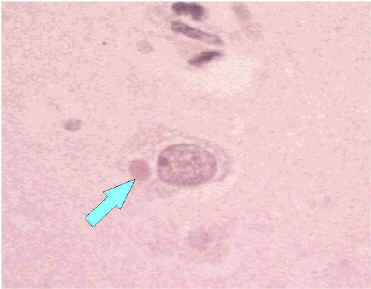
Negri body in infected neuron.
Sellers strain
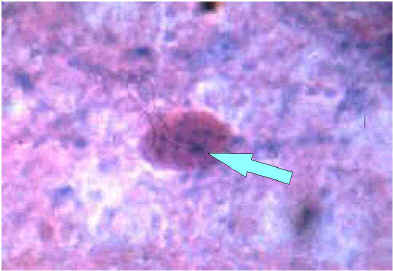
Enlargement of a Negri body in Sellers stained brain tissue. Note the basophilic (dark blue granules in the inclusion).
Immunohistochemistry (IHC)
IHC methods for rabies detection provide sensitive and specific means to detect rabies in formalin-fixed tissues. These methods are more sensitive than histologic staining methods, such as H&E and Sellers stains. Like the dFA test, these procedures use specific antibodies to detect rabies virus inclusions. The techniques use enzyme-labeling systems that increase sensitivity. In addition, monoclonal antibodies may be used to detect rabies virus variants.
Histologic section of brain from a rabid animal
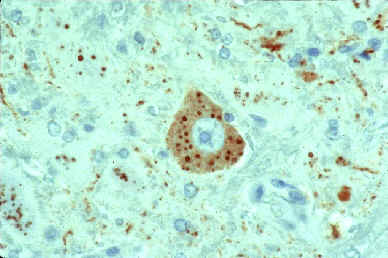
The slide shows a rabies virus-infected neuronal cell with intracytoplasmic inclusions. The red stain indicates areas of rabies viral antigen by using IHC or avidin-biotin complex (ABC) technique.
Ultrastructure
The ultrastructure of viruses can be examined by electron microscopy. Using this method, the structural components of viruses and their inclusions can be observed in detail. Rabies virus is in the family of Rhabdoviruses. When viewed with an electron microscope Rhabdoviruses are seen as bullet-shaped particles.
Negatively stained Rhabdovirus as seen through an electron microscope.
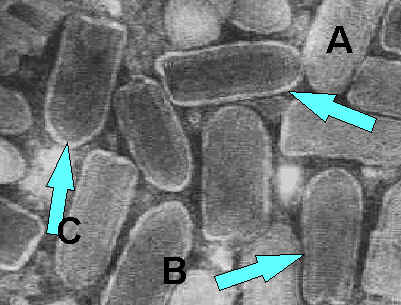
a) Notice the bullet shape of the virus. b) See the "bee hive" like striations of the RNP. c) Notice the glycoprotein spikes in the outer membrane bilayer.
Rabies virus budding from an inclusion (Negri body) into the endoplasmic reticulum in a nerve cell.
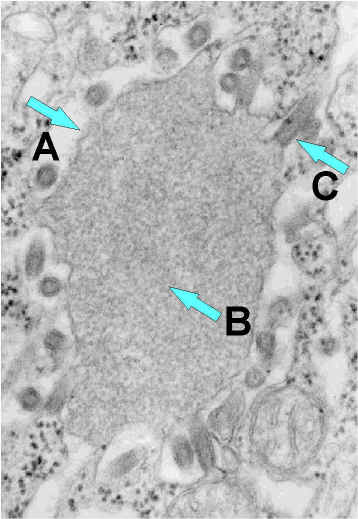
a) Negri body. b) Notice the abundant RNP in the inclusion. c) Budding rabies virus.
Ribonucleoprotein
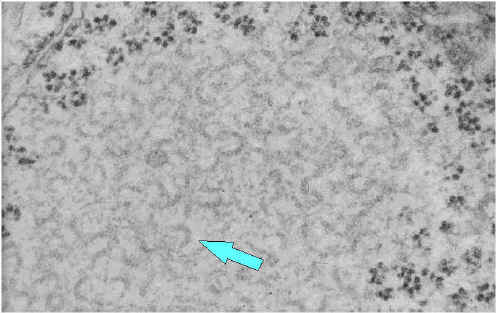
Notice the abundant strands of coiled RNP (almost everything in the image is RNP).
Amplification methods
Samples containing small amounts of rabies virus may be difficult to confirm as rabies-postive by routine methods. Virus isolation in cell cultures increases the virus concentration because the virus replicates in cell cultures. Mouse neuroblastoma cells (MNA) and baby hamster kidney (BHK) cells provide an excellent environment for amplification of rabies virus without the use of animals.
Another method for amplifying the nucleic acid portion of rabies virus uses biochemical methods. With this procedure, rabies virus RNA can be enzymatically amplified as DNA copies. Rabies RNA can be copied into a DNA molecule using reverse transcriptase (RT). The DNA copy of rabies can then be amplified using polymerase chain reaction (PCR). This technique can confirm dFA results and can detect rabies virus in saliva and skin biopsy samples.
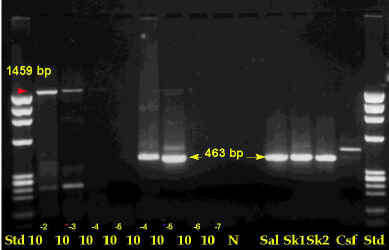
The figure shows PCR test results for rabies virus. The arrows indicate positions of positive bands.
Page last reviewed: December 1, 2003
Content Source: National Center for Zoonotic, Vector-Borne, & Enteric Diseases (ZVED)
