ISSN: 1080-6059
Volume 14, Number 1–January 2008
Dispatch
Molecular Epidemiology of Dengue Virus Strains from Finnish Travelers
Eili Huhtamo,* ![]() Nathalie Y. Uzcátegui,* Heli Siikamäki,†
Auli Saarinen,* Heli Piiparinen,* Antti Vaheri,* and Olli Vapalahti*‡§
Nathalie Y. Uzcátegui,* Heli Siikamäki,†
Auli Saarinen,* Heli Piiparinen,* Antti Vaheri,* and Olli Vapalahti*‡§
Eili Huhtamo,* Nathalie Y. Uzcátegui,* Heli Siikamäki,†
Auli Saarinen,* Heli Piiparinen,* Antti Vaheri,* and Olli Vapalahti*‡§
Suggested citation for this article
Abstract
We characterized 11 dengue virus (DENV) isolates obtained from Finnish travelers
during 2000–2005 using monoclonal antibodies and phylogenetic analysis.
The analysis of DENV isolated from travelers contributes to the global picture
of strain distribution and circulation. The isolates included all serotypes,
including a DENV-2 isolate from Ghana.
Dengue viruses (DENV 1–4) are mosquito-borne members of the family Flaviviridae, genus Flavivirus. Dengue is regarded as the most significant arboviral disease in the world. Disease incidence and prevalence are rising in dengue-endemic areas, and travelers are increasingly affected. The disease can vary from asymptomatic to febrile disease, classic dengue fever, or complications such as dengue hemorrhagic fever or dengue shock syndrome. Several virus- and host-specific factors have been suggested to correlate with severe disease outcomes, which are mostly associated with secondary infections (1). These outcomes are not common in European travelers, and deaths are rare (2). In recent years, the number of annually diagnosed cases has increased in Finland from an average of 10 to >20 in 2006 (Huhtamo et al., unpub. data). In the present study, study samples collected during 1999–2005 were studied by virus isolation. Virus isolates were not obtained from year 1999 samples; all isolates obtained from these samples were from the years 2000–2005.
The Study
Patients returning from dengue-endemic areas with fever and other symptoms compatible with dengue were treated mainly at university hospitals in Finland. Because of clinical suspicion, serum samples were tested for antibodies to DENV at Helsinki University Central Hospital Laboratory. The diagnosis was based on detection of immunoglobulin (Ig) M in the acute- or convalescent-phase sample or on a 4-fold IgG titer rise in paired serum specimens in an in-house IgG immunofluorescence assay (IFA), and IgM-enzyme immunoassay (Focus Technologies, Cypress, CA, USA). For this study, serum specimens from all patients were aliquoted and stored at –70ºC.
From patients with dengue diagnosis, acute-phase serum specimens with IgG titers <320 (IFA) were chosen for virus isolation (n = 40). Virus isolations were done simultaneously in 2 cell lines: in Vero E6 cells (ATCC CRL-1586) grown in minimal essential medium at 37°C and 5% CO2, and in C6/36 Aedes albopictus cells (ATCC CRL-1660) grown in Leibowitch L-15 medium at room temperature. Cells in 25-cm2 flasks were incubated with 50 μL of patient serum for 1 hour and observed for 24 days for cytopathic effects (CPEs). When CPEs were evident, cells were harvested for IFA, and RNA was extracted from supernatants for reverse transcriptase–PCR (RT-PCR). In the absence of CPEs, cells were subcultured after 7 days into 75-cm2 culture flasks and studied by IFA on days 7 and 24.
In IFA, the cells were stained with a DENV-positive serum and DENV–type-specific monoclonal antibodies (MAbs) (3). RNA was extracted from IFA- or CPE-positive culture supernatants with a Viral RNA Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer's instructions. RT-PCR targeting the capsid–premembrane (C-preM) region was performed using DENV-specific primers (4), Expand reverse transcriptase (Roche, Basel, Switzerland) and Taq DNA polymerase (Fermentas, Glen Burnie, MD, USA).
A total of 11 DENV strains were isolated from different geographic locations, including the 4 serotypes (DENV-1, n = 4; DENV-2, n = 2; DENV-3, n = 3; DENV-4, n = 2; Table). The serum samples yielding virus isolates were drawn within 1 week after onset of symptoms, which included fever, headache, muscular pain, rash, and nausea. Most of these samples were positive for antibodies to DENV (IgM positive, n = 8; IgG positive, n = 5).
Isolates were either strains that grew in both of the tested cell lines (n = 6) or strains that grew only in C6/36 cells (n = 5). Two of the DENV-3 isolates (2 and 7) were detectable considerably earlier in Vero E6 than in C6/36 cells. DENV-1 isolates showed 2 distinct growth patterns; isolates 4 and 8 grew only in C6/36 cells, and isolates 3 and 11 grew in both tested cell lines (Table).
All isolates were successfully serotyped with the RT-PCR of Lanciotti et al. (4), in agreement with results of the MAb IFA. However, isolate 3 (DENV-1) had particular properties in type-specific MAb IFA, depending on the cell type because it showed positive results in infected C6/36 cells and negative results in infected VE6 cells.
First-round RT-PCR amplicons were purified by using ExoSAP-IT (US Biochemicals, Cleveland, OH, USA), and directly sequenced. When necessary, the envelope gene was amplified using previously described primers (5) and sequenced. Nucleotide sequences of the isolates were aligned with published DENV sequences from GenBank (Appendix Table) using ClustalW (www.ebi.ac.uk/tools/clustalw). Phylogenetic analysis was performed by the neighbor-joining method with a Kimura 2-parameter model using MEGA3 software version 3.1 (6).
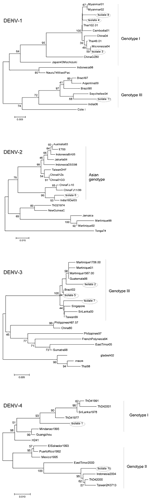 |
Figure 1. Neighbor-joining phylogenetic trees of the 4 dengue virus (DENV) serotypes based on the 454-bp capsid–premembrane (C-preM) region sequences obtained from first-round amplicons... |
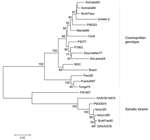 |
Figure 2. Neighbor-joining phylogenetic tree of dengue virus type 2 (DENV-2) based on the envelope gene sequence (1,485 bp). Isolate 9 from Ghana is circled. Bar represents nucleotide substitutions/site. |
Phylogenetic analyses (Figure 1) showed that isolates 3, 4, and 8 (DENV-1) clustered with Asiatic DENV-1 strains of genotype I (7), which corresponded with the patients' travel history. Isolate 11 (DENV-1) from India clustered with a genotype III strain isolated a year earlier from the Seychelles. Isolate 6 (DENV-2), obtained from Sri Lanka in 2003, clustered with a strain isolated in the same year from India. Unlike the other isolates, isolate 9 (DENV-2), obtained in Ghana in 2005, did not group with any of the representative strains of the C-preM region, for which no African sequences were available in GenBank. The additionally studied envelope gene sequence grouped with previous African isolates of the cosmopolitan genotype (8) (Figure 2).
The DENV-3 isolates represented genotype III (9) (Figure 1). Isolate 2 from Cuba clustered with strains from Martinique in agreement with previous data on Cuban strains (10). Isolate 7 (DENV-3), obtained in Sri Lanka in 2004, clustered with strains from Singapore, Sri Lanka, and Taiwan. Isolate 5 was identical in sequence to a strain isolated 1 year earlier from a patient in Brazil who died (11). DENV-4 isolates represented 2 different genotypes; isolate 1 from Sri Lanka clustered with genotype I strains, and isolate 10 from Indonesia clustered with genotype II (12).
Conclusions
Studies on imported DENV have provided interesting insights to the global picture of circulating strains (13,14), and also have led to the discovery of novel DENV strains and lineages (15,16). In this study, we characterized 11 strains of DENV isolated from Finnish travelers in 2000–2005 and provided new information about strains circulating in India, Sri Lanka, and Ghana.
Previous studies have shown that DENV isolation is possible when antibody levels are low (17). However, in this study, most samples yielding virus isolates were antibody positive. The patients had primary infections, except for 1 patient, who had an IgG titer of 320 in the acute phase, which is suggestive of a secondary infection. This was the only patient with any bleeding symptoms, i.e., prolonged bleeding from the venopuncture site.
Virus isolates from Finnish travelers were heterogeneous. All patients had dengue fever, including the patient whose isolate was identical in sequence to a strain isolated from a patient who had died. Since the disease outcomes of the patients were uneventful, no associations could be made between the infective virus serotype or strain and disease severity.
Both mammalian and mosquito cells were used in virus isolation, which enabled the detection of other flaviviruses that may have caused seropositivity through cross-reaction. All DENV isolates grew in C6/36 mosquito cells; however, use of 2 cell lines showed variation in the growth patterns of the isolates in different cell types. We observed that some DENV-3 strains were detectable earlier in mammalian Vero E6 cells than in C6/36 cells, which suggested a different capability to infect these cells. This property could not be associated with pathogenicity in this study; thus, the biologic relevance of this phenomenon is unknown.
The DENV type-specific MAb IFA showed that one of the DENV-1 isolates (isolate 3) had distinct antigenic properties when cultured in mammalian or mosquito cells. Whether this strain represents MAb-escape properties requires further studies.
The phylogenetic grouping of the isolates was consistent with the travel history of the patients in most cases. However, isolate 11 (DENV-1) from India clustered with a genotype III strain isolated a year earlier from the Seychelles, which suggested strain transfer between these countries.
Phylogenetic analysis of isolate 9 (Ghana 2005) showed that it could be grouped with other African isolates of the cosmopolitan genotype (Figure 2). To our knowledge, this is the first DENV-2 strain characterized from Ghana (the geographically nearest isolate is from Burkina Faso in 1983). This grouping demonstrates sustained circulation of DENV-2 strains in Africa for decades.
The 11 DENV isolates represent a random sample from different geographic locations. Three strains were isolated from travelers returning from Sri Lanka, first in 2000 (DENV-4), followed by isolates in 2003 (DENV-2) and 2004 (DENV-3). These strains demonstrate extensive DENV serotype cocirculation.
Acknowledgments
We thank Ernest Gould for providing the MAbs used in this study; Jukka Lumio, Jarmo Oksi, Irma Koivula, and Kari Sammalkorpi for providing clinical patient data; and Raija Leveelahti, Johanna Myllynen, Essi Hasu, Minna Ulmanen, and Kirsti Räihä for technical assistance.
This study was supported by grants from Hospital District of Helsinki and Uusimaa (TYH4211, TYH6215) and National Technology Agency (TEKES).
Ms Huhtamo is a PhD candidate at Helsinki Biomedical Graduate School and conducts research at the Department of Virology, Haartman Institute, University of Helsinki. Her research involves mosquito-borne flaviviruses.
References
- Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42.
- Wichmann O, Gascon J, Schunk M, Puente S, Siikamaki H, Gjorup I, et al. European network on surveillance of imported infectious diseases. Severe dengue virus infection in travelers: risk factors and laboratory indicators. J Infect Dis. 2007;195:1089–96.
- Henchal EA, McCown JM, Seguin MC, Gentry MK, Brandt WE. Rapid identification of dengue virus isolates by using monoclonal antibodies in an indirect immunofluorescence assay. Am J Trop Med Hyg. 1983;32:164–9.
- Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51.
- Uzcategui NY, Camacho D, Comach G, Cuello de Uzcategui R, Holmes EC, Gould EA. evolution and recombination. J Gen Virol. 2001;82:2945–53.
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–63.
- A-Nuegoonpipat A, Berlioz-Arthaud A, Chow V, Endy T, Lowry K, Mai le Q, et al. Sustained transmission of dengue virus type 1 in the Pacific due to repeated introductions of different Asian strains. Virology. 2004;329:505–12.
- Twiddy SS, Farrar JJ, Vinh Chau N, Wills B, Gould EA, Gritsun T, et al. Phylogenetic relationships and differential selection pressures among genotypes of dengue-2 virus. Virology. 2002;298:63–72.
- Messer WB, Gubler DJ, Harris E, Sivananthan K, de Silva AM. Emergence and global spread of a dengue serotype 3, subtype III virus. Emerg Infect Dis. 2003;9:800–9.
- Miagostovich MP, dos Santos FB, Fumian TM, Guimaraes FR, da Costa EV, Tavares FN, et al. Complete genetic characterization of a Brazilian dengue virus type 3 strain isolated from a fatal outcome. Mem Inst Oswaldo Cruz. 2006;101:307–13.
- Rodriguez-Roche R, Alvarez M, Holmes EC, Bernardo L, Kouri G, Gould EA, et al. Dengue virus type 3, Cuba, 2000–2002. Emerg Infect Dis. 2005;11:773–4.
- Klungthong C, Zhang C, Mammen MP Jr, Ubol S, Holmes EC. The molecular epidemiology of dengue virus serotype 4 in Bangkok, Thailand. Virology. 2004;329:168–79.
- Teichmann D, Rogler G, Grobusch MP, Schuler-Maue W, Klein E. Imported dengue virus type 2 infection acquired during an outbreak in India. Eur J Clin Microbiol Infect Dis. 1999;18:310–2.
- Ito M, Yamada K, Takasaki T, Pandey B, Nerome R, Tajima S, et al. Phylogenetic analysis of dengue viruses isolated from imported dengue patients: possible aid for determining the countries where infections occurred. J Travel Med. 2007;14:233–44.
- Nukui Y, Tajima S, Kotaki A, Ito M, Takasaki T, Koike K, et al. Novel dengue virus type 1 from travelers to Yap State, Micronesia. Emerg Infect Dis. 2006;12:343–6.
- Domingo C, Palacios G, Jabado O, Reyes N, Niedrig M, Gascon J, et al. Use of a short fragment of the C-terminal E gene for detection and characterization of two new lineages of dengue virus 1 in India. J Clin Microbiol. 2006;44:1519–29.
- Yamada K, Takasaki T, Nawa M, Kurane I. Virus isolation as one of the diagnostic methods for dengue virus infection. J Clin Virol. 2002;24:203–9.
Figures
Figure 1. Neighbor-joining phylogenetic trees of the 4 dengue virus (DENV)
serotypes based on the 454-bp capsid–premembrane (C-preM) region sequences
obtained from first-round amplicons...
Figure 2. Neighbor-joining phylogenetic tree of dengue virus type 2 (DENV-2)
based on the envelope gene sequence (1,485 bp). Isolate 9 from Ghana is circled.
Bar represents nucleotide substitutions/site.
Tables
Table. Dengue virus isolates from Finnish travelers, 2000–2005
Appendix Table. Dengue virus isolates from Finnish travelers, 2000–2005
Suggested Citation for this Article
Huhtamo E, Uzcátegui NY, Siikamäki H, Saarinen A, Piiparinen H, Vaheri A, et al. Molecular epidemiology of dengue virus strains from Finnish travelers. Emerg Infect Dis [serial on the Internet]. 2008 Jan [date cited]. Available from http://www.cdc.gov/EID/content/14/1/80.htm
Please use the form below to submit correspondence to the authors or contact them at the following address:
Eili Huhtamo, Haartman Institute, Department of Virology (Haartmaninkatu 3), PO Box 21, 00014, University of Helsinki, Helsinki, Finland; email: eili.huhtamo@helsinki.fi
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted December 21, 2007
This page last reviewed December 21, 2007
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435