|
|
||||||||||||||||
|
|
|
|
|
|||||||||||||
|
|
|
|
Vaccine Safety Datalink (VSD) Project
Topics on This Page:
The Vaccine Safety Datalink (VSD) project is a collaborative effort between CDC's Immunization Safety Office and eight large managed care organizations (MCO). The VSD project was established in 1990 to monitor immunization safety and address the gaps in scientific knowledge about rare and serious adverse events following immunization. The VSD project includes a large linked database that uses administrative data sources at each MCO. Each participating site gathers data on vaccination (vaccine type, date of vaccination, concurrent vaccinations), medical outcomes (outpatient visits, inpatient visits, urgent care visits), birth data, and census data. The VSD project allows for planned immunization safety studies as well as timely investigations of hypotheses that arise from review of medical literature, reports to the Vaccine Adverse Event Reporting System (VAERS), changes in immunization schedules, or the introduction of new vaccines. Since 1990, investigators from the VSD project have published more than 75 scientific articles. The VSD project has addressed the following immunization safety concerns:
The VSD project has proven to be a highly effective tool for evaluating immunization safety. Recent ResearchCDC studied the risk of febrile (fever-related) seizures among children who received the combined measles, mumps, rubella, and varicella (MMRV) vaccine, compared with children who received two separate vaccines: one for measles, mumps, and rubella (MMR) and one for varicella (chickenpox).
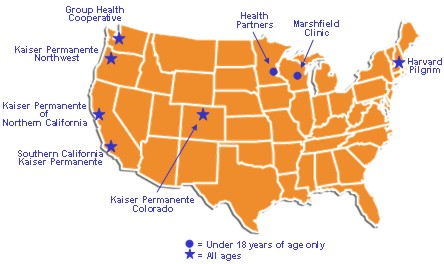
The Infant and Environmental Exposures to Thimerosal and Neuropsychological Outcomes at Ages 7 to 10 Years study investigated possible association between prenatal and/or early childhood exposure to thimerosal-containing vaccines and/or immunoglobulins and neuropsychological functioning at ages seven to ten years. Managed Care Organization Sites
Objectives
Priority Studies
Rapid Cycle Analysis (RCA)Rapid Cycle Analysis (RCA) is an active surveillance system designed to detect adverse events (possible side effects) following vaccination in near real time, so the public can be informed quickly of possible risks. Read the VSD 2004–2005 Annual Report.* *Links to non-Federal organizations found at this site are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization Web pages found at these links.
Page last reviewed: April 18, 2008
Page last updated: April 18, 2008 Content source: Immunization Safety Office, Office of the Chief Science Officer | |||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||
|