Can We Help the Ear Repair Itself?
Scientists hope to “re-start” the ear’s developmental machinery
NIDCD-supported scientists are identifying the genes necessary for forming the ears and enabling them to detect sound. They hope that a good understanding of normal development will enable them to correct or prevent hearing and balance disorders in children. As the Baby Boomer generation gets older, scientists hope their knowledge will help them develop therapies to restore hearing and balance lost due to infection, injury, noise, and the aging process.
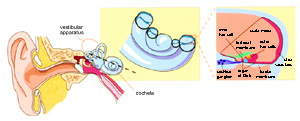
Schematic illustration of the mammalian auditory system (from Griffith and Friedman, Nature Genetics, 1999). View larger image.
How does the ear detect sound? Auditory hair cells are a vital part of the ear’s ability to detect sound. Located in the inner ear, hair cells have microscopic hair-like projections that protrude from their tops. The surfaces of these hairs contain pores, or channels, that open after the hair-like projections are subjected to mechanical force. Inside the ear, the force comes from a sound wave that moves through the fluid underlying the hair cells, lifts them up, and drives them into an overlying membrane. The channels open and release small molecules in a chemical chain reaction that ultimately tells the auditory nerve to tell the brain that sound has been detected.
Signals Controlling Hair Cell Development. In 1993, five scientists at the University of Virginia wrote a review article addressing an important question for developmental biologists: how do the ears take shape from an identical mass of unspecified cells to an organized pattern of hair cells and supporting cells? Cutting-edge research in simple models like the fruit fly described how a system called “lateral inhibition” is used to specify different kinds of cells, such as neurons and non-neuronal cells in other developing systems. Based on this, the group proposed that mammalian ears use a similar system of organization.
The lateral inhibition theory proposes that individual cells begin to take on the identity of one cell type, such as a nerve cell. The developing nerve cells, or neurons, then inhibit neighboring cells (located at their sides, or laterally) from also becoming neurons. This theory explains how a group of identical cells can develop into a mixture of neurons surrounded by non-neurons.
Scientists began using lateral inhibition as a framework for posing questions about how the ears develop. This approach has led to the discovery of some very important mechanisms determining how mammalian ears organize themselves – and these basic details of development may one day show scientists how to help individuals with hearing loss to hear sound once again.
If an auditory hair cell is keeping its neighbors from becoming hair cells, then destroying it should result in loss of the inhibition. That is, the neighboring cells may then become hair cells in its place. That was exactly what graduate student Matthew Kelley observed in 1995, when he used a laser to destroy selected mouse auditory hair cells. After the hair cells were destroyed, time lapse photos showed neighboring supporting cells migrating into the hair cell layer and developing as new hair cells. Although these studies suggested that lateral inhibition was indeed at work, nobody yet knew what genes might be involved in this process. Once again, a breakthrough came through use of the fruit fly as a model for investigation.
Molecular Detective Work: From Flies to Mice. Fruit flies use a primitive structure called the chordotonal organ to detect sound, and mutant flies missing a gene called atonal lack these organs. Scientists began searching for a gene similar to Atonal (an ortholog) that is responsible for mammalian ear development. They discovered that a candidate gene, called Math1, or Atoh1, was able to restore chordotonal organs in atonal mutant flies. When NIDCD-supported scientists “knocked out” or removed the Math1 gene, the mutant mice suffered a wide range of problems, including deafness and balance problems. In 2002, a different group of NIDCD-supported scientists reported that the fruit fly gene can “rescue” or prevent the defects of mice lacking the Math1 gene. This research demonstrated an amazing similarity between the mouse and fruit fly auditory systems, and also established the importance of Math1 in mouse ear development.
Encouraged by these fly/mouse similarities, one laboratory searched for more fruit fly gene orthologs in the mouse ear. The group was led by Matthew Kelley, now heading up his own laboratory in the NIDCD intramural program. Fruit fly neurons express a gene called delta during lateral inhibition. In a 1999 publication, the laboratory reported that a mouse ortholog of delta, known as Jag2, was expressed in the right place and at the right time to play a role in lateral inhibition in mouse hair cell specification. Mutant mice that lack Jag2 produce far too many hair cells – more evidence that lateral inhibition is at work.
In 2005, the Kelley lab reported that not only do developing hair cells inhibit their neighbors from becoming hair cells, but also send signals that recruit nearby cells to develop as supporting cells. This means that if scientists use these genes to try to stimulate the development of extra hair cells (to replace those missing or damaged), they will not “use up” all the supporting cells, as had been feared. Each group of developing hair cells appears to organize itself in an appropriate pattern and to recruit the supporting cell types needed to maintain the group’s sound-detecting function.
Also in 2005, NIDCD-supported scientists at Harvard University, Northwestern University, Tufts, and the University of Virginia identified a gene that prevents the regeneration of hair cells. The group knew that a gene called “retinoblastoma” or Rb1, forces cells to exit the so-called “cell cycle,” meaning that they stop dividing and begin to mature. They also knew that Rb1 is expressed in mouse ears during the time they are generating hair cells. Based on this knowledge, they looked at the number of hair cells in mice lacking Rb1, and found that they have extra hair cells. Blocking Rb1 expression in cultured hair cells, they reactivated the cell cycle – causing the hair cells to begin dividing once again. This critical discovery provides scientists with a more detailed understanding of the genes controlling the development of hearing. If we want to make more hair cells, we must both activate hair cell “On” genes, and inactivate hair cell “Off” genes – such as Rb1.
Working to Restore Hearing. If an individual’s hearing loss is due to hair cell death, the only way to restore normal hearing is to replace the lost hair cells. However, under normal circumstances, mammals cannot replace hair cells. Due to years of intense and dedicated basic research, we now know some of the many genes important for ear development and hearing. How can we use this information to help those who are deaf?
Hijacking a Virus for a Good Cause – Gene Therapy. Viruses that have been altered to remove disease-causing elements and to include a gene of interest are a useful way for scientists to “deliver” genes to cells and tissues – the virus passes the inserted gene along when it infects the tissue. NIDCD-supported scientists at the University of Michigan used their knowledge of the ear’s gene expression programs to recruit cells in a deaf ear to become hair cells. They treated deafened guinea pig ears with a virus carrying the gene Math1/Atoh1, and found evidence that new hair cells were generated. More importantly, the treated animals showed functional evidence of partial restoration of their hearing. This is the first successful demonstration of gene therapy that improves hearing in formerly deaf animals. Scientists hope to one day use this type of gene therapy to restore hearing in humans.
Improving Gene Therapy Methods - Cow Virus Is Powerful New Tool. Even though scientists can use viruses to deliver important genes to the ear, sensory tissue of the ear is particularly challenging to infect. The viruses currently used are able to infect only a relatively small number of cells. NIDCD and NICHD intramural scientists collaborated to develop a new type of virus to deliver important genes to ear tissues. In 2005, the group reported that they had successfully used a modified cow virus to deliver genes to the ear tissues of rats. The virus infected nearly all hair cells and supporting cells, making it an attractive new tool for recruiting new hair cells within a damaged ear.
Scientists hope to use their combined knowledge of the ear’s developmental program and gene therapy techniques to restore hearing to those who become deaf. Such important scientific advances are based upon scientists’ ability to build on past discoveries in a wide ranging collection of animal model systems.
Citations: Di Pasquale G, Rzadzinska A, Schneider ME, Bossis I, Chiorini JA, Kachar B, A novel bovine virus efficiently transduces inner ear neuroepithelial cells. Mol Ther 11: 849-855, 2005.
Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y, Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med 11: 271-276, 2005.
Woods C, Montcouquiol M, Kelley MW, Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci 7: 1310-1318, 2004.
Top