|
"Estrogens" are a family of related molecules that stimulate the development and maintenance of female characteristics and sexual reproduction.
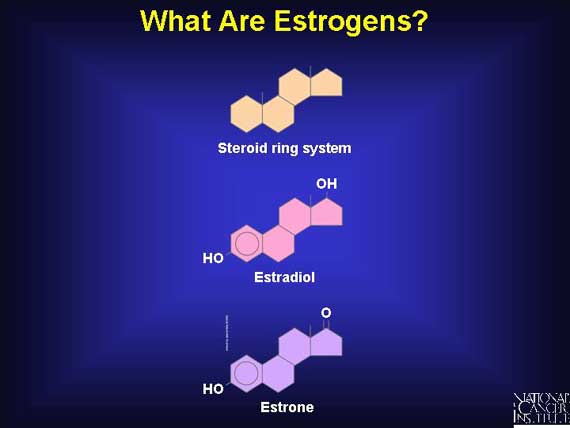
The natural estrogens produced by women are steroid molecules, which means that they are derived from a particular type of molecular skeleton containing four rings of carbon atoms, giving the shape shown here. The most prevalent forms of human estrogen are estradiol and estrone. Both are produced and secreted by the ovaries, although estrone is also made in the adrenal glands and other organs.
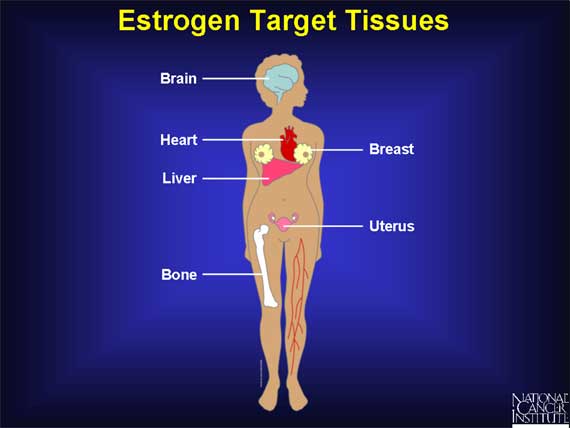
Estrogens are hormones, which means that they function as signaling molecules. A signaling molecule exerts its effects by traveling through the bloodstream and interacting with cells in a variety of target tissues.
The breast and the uterus, which play central roles in sexual reproduction, are two of the main targets of estrogen. In addition, estrogen molecules act on the brain, bone, liver, and heart.
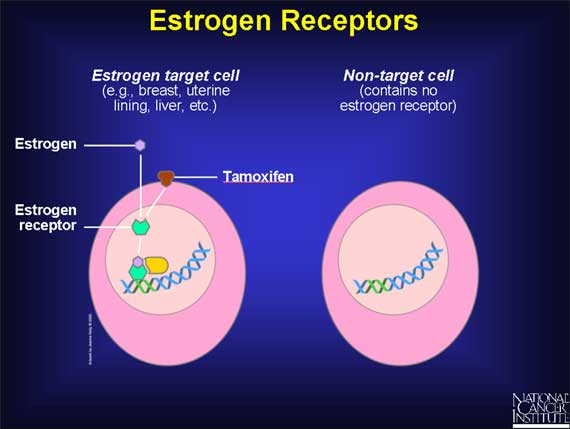
Estrogens act on target tissues by binding to parts of cells called estrogen receptors.
An estrogen receptor is a protein molecule found inside those cells that are targets for estrogen action. Estrogen receptors contain a specific site to which only estrogens (or closely related molecules) can bind.
The target tissues affected by estrogen molecules all contain estrogen receptors; other organs and tissues in the body do not. Therefore, when estrogen molecules circulate in the bloodstream and move throughout the body, they exert effects only on cells that contain estrogen receptors.
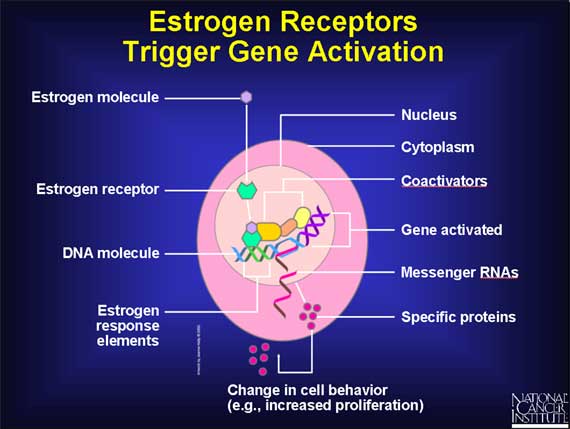
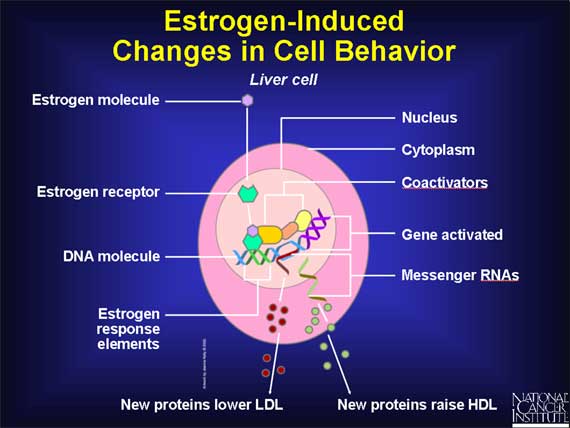
Estrogen receptors normally reside in the cell's nucleus, along with DNA molecules.
In the absence of estrogen molecules, these estrogen receptors are inactive and have no influence on DNA (which contains the cell's genes). But when an estrogen molecule enters a cell and passes into the nucleus, the estrogen binds to its receptor, thereby causing the shape of the receptor to change. This estrogen-receptor complex then binds to specific DNA sites, called estrogen response elements, which are located near genes that are controlled by estrogen.
After it has become attached to estrogen response elements in DNA, this estrogen-receptor complex binds to coactivator proteins and more nearby genes become active. The active genes produce molecules of messenger RNA, which guide the synthesis of specific proteins. These proteins can then influence cell behavior in different ways, depending on the cell type involved.
In liver cells, for example, estrogen alters the production of proteins that influence cholesterol levels in the blood.
Cholesterol does not readily dissolve in blood, so before it can be transported through the body, it first becomes bound to special cholesterol-carrying proteins called lipoproteins. The liver produces two such lipoproteins, called low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL-cholesterol is considered to be the "bad" form of cholesterol because it tends to release cholesterol directly onto the inner wall of arteries, creating the "plaque" that can lead to heart disease. In contrast, HDL is considered to be the "good" form of cholesterol because it inhibits the formation of plaque and carries cholesterol away from the arteries and back to the liver.
The net effect of estrogen's action on liver cells is to increase the amount of HDL cholesterol and to decrease the amount of LDL cholesterol. By increasing HDL and decreasing LDL, estrogen helps to lower the risk of heart disease.
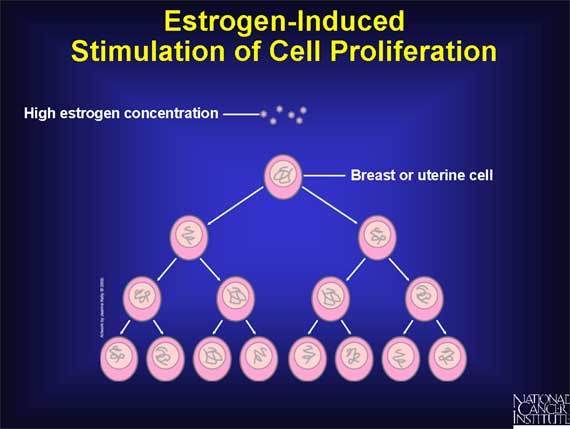
In some target tissues, the main effect of estrogen is to cause cells to grow
and divide, a process called cell proliferation.
In breast tissue, for example, estrogen triggers the proliferation of cells
lining the milk glands, thereby preparing the breast to produce milk if the
woman should become pregnant.
Estrogen also promotes proliferation of the cells that form the inner lining,
or endometrium, of the uterus, thereby preparing the uterus for possible
implantation of an embryo. During a normal menstrual cycle, estrogen levels
fall dramatically at the end of each cycle if pregnancy does not occur. As a
result, the endometrium disintegrates and is shed from the uterus and vagina in
a bleeding process called menstruation.
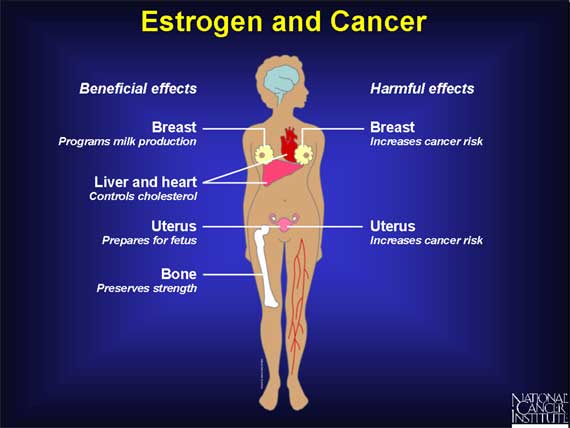
Paradoxically, estrogen can be both a beneficial and a harmful molecule.
The main beneficial effects of estrogen include its roles in
- programming the breast and uterus for sexual reproduction,
- controlling cholesterol production in ways that limit the buildup of plaque in the coronary arteries, and
- preserving bone strength by helping to maintain the proper balance between bone buildup and breakdown.
Unfortunately, in addition to these important beneficial effects, estrogen can also be harmful. The most serious problem arises from the ability of estrogen to promote the proliferation of cells in the breast and uterus. Although this ability to stimulate cell proliferation is one of estrogen's normal roles, it can also increase a woman's chance of developing breast or uterine cancer.
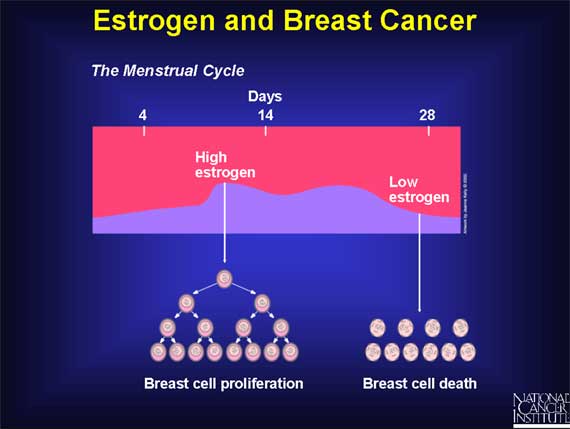
During each menstrual cycle, estrogen normally triggers the proliferation of cells that form the inner lining of the milk glands in the breast.
If pregnancy does not occur, estrogen levels fall dramatically at the end of each monthly menstrual cycle. In the absence of high estrogen levels, those milk gland cells that have proliferated in any given month will deteriorate and die, followed by a similar cycle of cell proliferation and cell death the following month. For the average woman, this means hundreds of cycles of breast cell division and cell death repeated over a span of roughly 40 years, from puberty to menopause.
But how do these estrogen-induced cycles of breast cell proliferation increase the risk of developing cancer?
Cancer is caused by DNA damage (i.e., mutations) in genes that regulate cell growth and division.
Some mutations are inherited, while others are caused by exposure to radiation or to mutation-inducing chemicals such as those found in cigarette smoke. Mutations also can occur spontaneously as a result of mistakes that are made when a cell duplicates its DNA molecules prior to cell division.
When cells acquire mutations in specific genes that control proliferation, such as proto-oncogenes or tumor suppressor genes, these changes are copied with each new generation of cells. Later, more mutations in these altered cells can lead to uncontrolled proliferation and the onset of cancer. (For more information on how gene mutations cause cancer, see Understanding Cancer.)
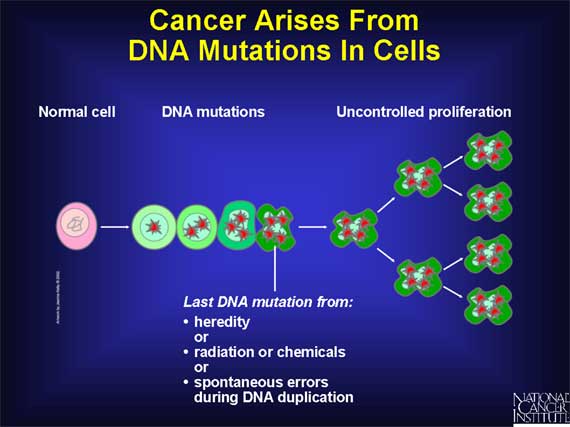
Although estrogen does not appear to directly cause the DNA mutations that
trigger the development of human cancer, estrogen does stimulate cell
proliferation.
Therefore, if one or more breast cells already possesses a DNA mutation that
increases the risk of developing cancer, these cells will proliferate (along
with normal breast cells) in response to estrogen stimulation. The result will
be an increase in the total number of mutant cells, any of which might
thereafter acquire the additional mutations that lead to uncontrolled
proliferation and the onset of cancer.
In other words, estrogen-induced cell production leads to an increase in the
total number of mutant cells that exist. These cells are at increased risk of
becoming cancerous, so the chances that cancer may actually develop are
increased.
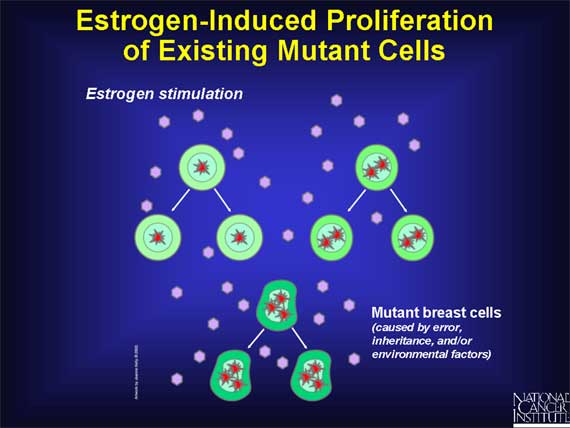
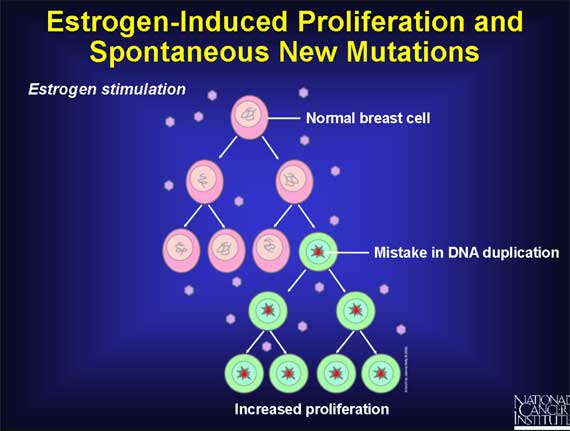
Even in women who do not have any mutant breast cells, estrogen-induced proliferation of normal breast cells may still increase the risk of developing cancer.
The reason involves DNA. A cell must duplicate its DNA molecules prior to each cell division, thereby ensuring that the two new cells resulting from the process of cell division each receive one complete set of DNA molecules. But the process of DNA duplication occasionally makes mistakes, so the resulting DNA copies may contain a small number of errors (i.e., mutations). If one of these spontaneous mutations occurs in a gene that controls cell growth and division, it could lead to the development of cancer.
Proliferation of normal cells from exposure to estrogen creates a vulnerability to spontaneous mutations, some of which might represent a first step on the pathway to cancer.
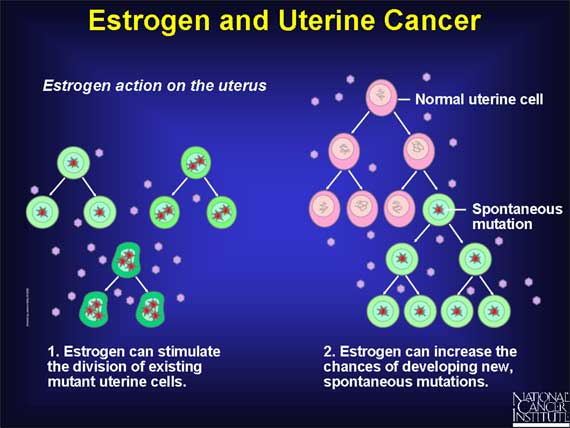
In the uterus, estrogen triggers the proliferation of endometrial lining cells during each month of the menstrual cycle, followed by death of these cells during menstruation. Over a span of 40 years, from puberty to menopause, hundreds of cycles of cell division and cell death will occur.
These repeated cycles of estrogen-induced cell division tend to increase the risk of developing cancer in the same two ways as in the breast: Estrogen can stimulate the division of uterine cells that already have DNA mutations, and it also increases the chances of developing new, spontaneous mutations when estrogen stimulates cell proliferation. Whether the mutations are inherited or spontaneous, estrogen-driven proliferation will increase the number of these altered cells that can ultimately lead to the development of uterine cancer.
Since estrogen can promote the development of cancer in the breast and uterus, it seems logical to postulate that substances that block the action of estrogen might be helpful in preventing or treating these two types of cancer.
This rationale has led scientists to work on the development of "antiestrogen" drugs that can block the action of estrogens and thereby interfere with, or even prevent, the proliferation of breast and uterine cancer cells. Antiestrogens work by binding to estrogen receptors, blocking estrogen from binding to these receptors. This also blocks estrogen from activating genes for specific growth-promoting proteins.
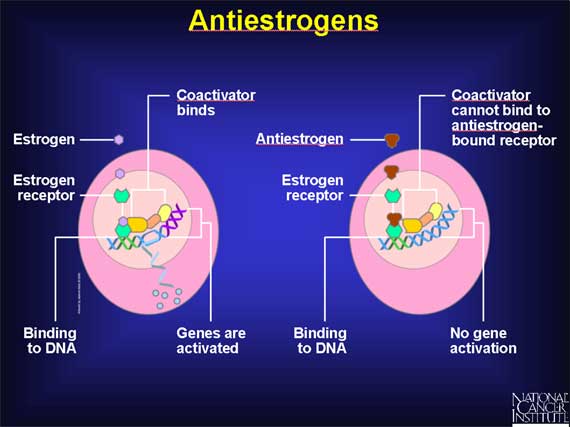
In working on the development of antiestrogens, scientists have made a somewhat
surprising discovery. Some drugs that block the action of estrogen in certain
tissues actually can mimic the action of estrogen in other tissues.
Such selectivity is made possible by the fact that the estrogen receptors of
different target tissues vary in chemical structure. These differences allow
estrogen-like drugs to interact in different ways with the estrogen receptors
of different tissues. Such drugs are called selective estrogen receptor
modulators, or SERMs, because they selectively stimulate or inhibit the
estrogen receptors of different target tissues. For example, a SERM might
inhibit the estrogen receptor found in breast cells but activate the estrogen
receptor present in uterine endometrial cells. A SERM of this type would
inhibit cell proliferation in breast cells, but stimulate the proliferation of
uterine endometrial cells.
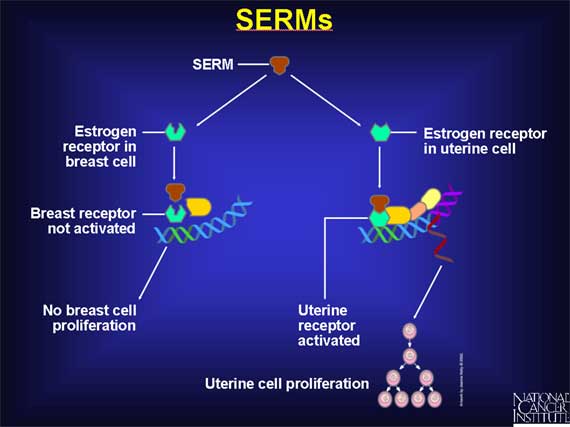
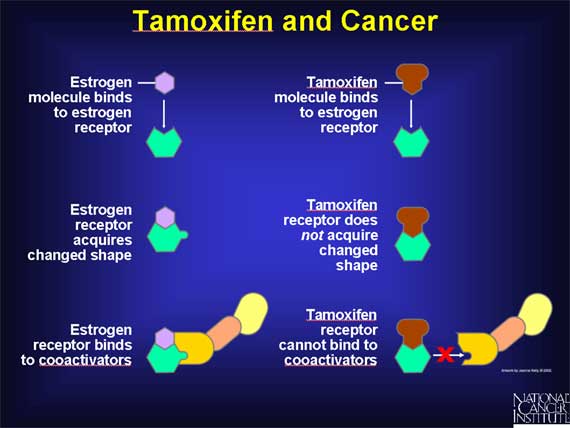
The first SERM to be investigated extensively for its anticancer properties is a drug called tamoxifen.
Tamoxifen blocks the action of estrogen in breast tissue. Tamoxifen exerts this antiestrogenic effect by binding to the estrogen receptors of breast cells, thereby preventing estrogen molecules from binding to these receptors. But unlike the normal situation, when estrogen binds to its receptor, the binding of tamoxifen to the receptor does not cause the receptor molecule to acquire the changed shape that allows it to bind to coactivators. As a result, the genes that stimulate cell proliferation cannot be activated.
By interfering with estrogen receptors in this way, tamoxifen blocks the ability of estrogen to stimulate the proliferation of breast cells.
In women who have breast cancer, proliferation of the breast cancer cells is
often driven by estrogen, just as in the case of normal breast cells.
Since tamoxifen can block the effects of estrogen on breast cells, scientists
predicted that breast cancer could be treated by using tamoxifen to interfere
with estrogen-induced cell proliferation. Based on encouraging results obtained
in experimental trials, tamoxifen was first approved for such use in breast
cancer treatment in the 1970s.
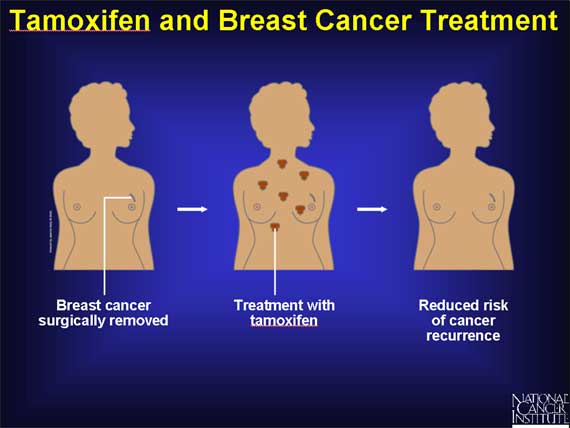
The first step in treating women with breast cancer is to surgically remove the
cancer from the breast. It is difficult to be certain that every cancer cell
has been removed at the time of surgery because some breast cancer cells could
have spread to surrounding tissues or other organs prior to the operation.
Therefore, women often receive some type of treatment after surgery (adjuvant
therapy) to prevent the growth of any cancer cells that might remain in the
body. Studies show that when tamoxifen is used for this purpose, the risk of
cancer recurrence is reduced.
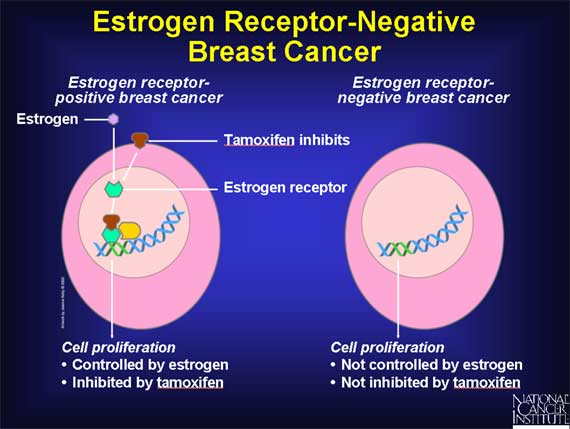
Unlike normal breast cells, cancer cells arising in the breast do not always have receptors for estrogen.
Breast cancers that DO have estrogen receptors are said to be "estrogen receptor-positive," while those breast cancers that DO NOT possess estrogen receptors are "estrogen receptor-negative." In women with estrogen receptor-positive cancers, cancer cell growth is under the control of estrogen. Therefore, such cancers are often susceptible to treatment with tamoxifen, because tamoxifen works by blocking the interaction between estrogen and the estrogen receptor.
In contrast, the growth of estrogen receptor-negative cancer cells is not governed by estrogen, or treated with tamoxifen.
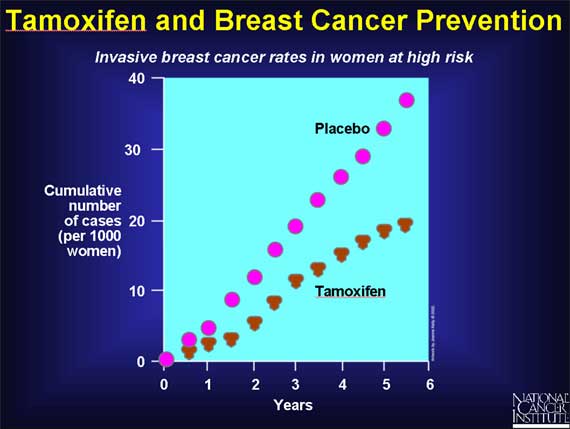
Once tamoxifen had been shown to reduce the risk of cancer reappearing after breast cancer surgery (in women with estrogen receptor-positive cancers), the question arose whether tamoxifen might also be helpful in preventing breast cancer in women at high risk of developing the disease.
The rationale for using tamoxifen to try to prevent breast cancer is similar to the rationale for using tamoxifen in breast cancer treatment. Estrogen-induced cycles of breast cell proliferation increase a woman's risk of developing breast cancer. Therefore, using tamoxifen to block the action of estrogen in the breast in healthy women might be expected to decrease a woman's chances of developing cancer in the future.
In 1992, the National Cancer Institute opened a study involving more than 13,000 healthy women considered to be at high risk for breast cancer based on their family or medical history. Half the women were given tamoxifen, while the other half were given a placebo. After 5 years, the group receiving tamoxifen had a lower rate of breast cancer.
Although tamoxifen has been useful both in treating breast cancer patients and in decreasing the risk of getting breast cancer in women at high risk, it also has some serious side effects.
These side effects arise from the fact that while tamoxifen acts as an antiestrogen that blocks the effects of estrogen on breast cells, it mimics the actions of estrogen in other tissues such as the uterus. Its estrogen-like effects on the uterus stimulate proliferation of the uterine endometrium and increase the risk of uterine cancer.
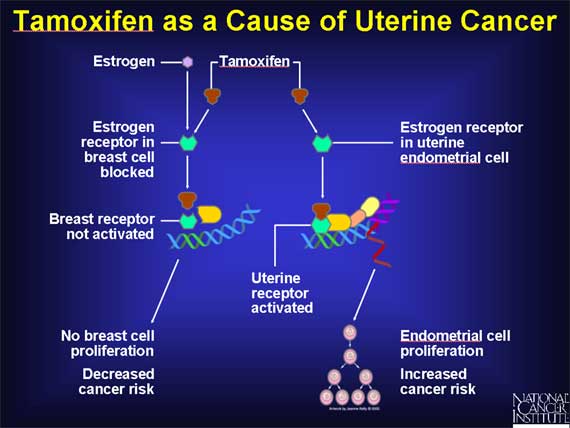
The fact that tamoxifen blocks the action of estrogen in breast tissue while mimicking the action of estrogen in the uterus means that it functions as a SERM, selectively blocking or stimulating the estrogen receptors of different target tissues.
In addition to acting like estrogen in the uterus, tamoxifen resembles estrogen in its ability to lower LDL cholesterol levels. And in postmenopausal women, tamoxifen also resembles estrogen in its ability to preserve or increase bone density. Thus, aside from its tendency to increase the risk of uterine cancer, tamoxifen has a number of potentially beneficial properties.
As a result, scientists have been actively working on the development of other SERMs that might exhibit some of the beneficial properties of tamoxifen without sharing its potentially harmful effects.
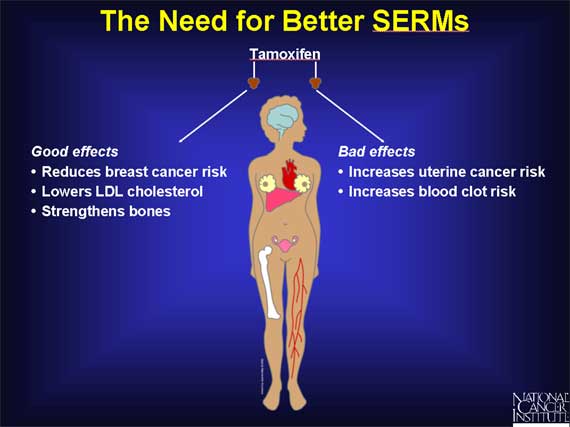
In addition to their relevance for cancer treatment and prevention, SERMs may also be potentially important for women who have passed through menopause and, therefore, produce little estrogen.
The lack of estrogen in postmenopausal women is linked to several health problems. For example, estrogen has positive effects on blood vessels and on bones. After menopause, though, women are at increased risk for heart disease and for osteoporosis, a weakening of the bones that causes them to become more vulnerable to fractures.
To counteract these potential problems, many postmenopausal women take hormone pills containing estrogen to strengthen bones and help control other menopausal symptoms. But as a consequence, such women may also subject themselves to the harmful effects of estrogen, namely, an increased risk for invasive breast cancer and for uterine cancer.
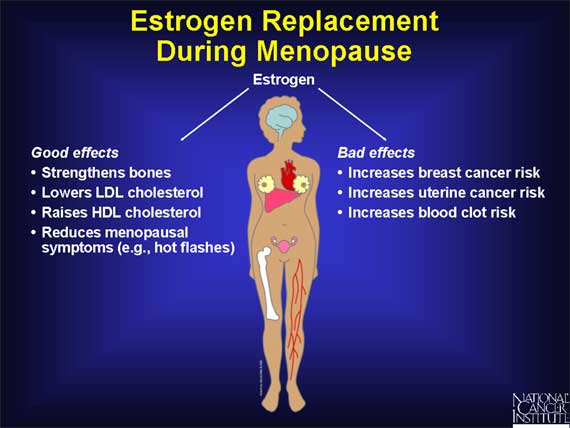
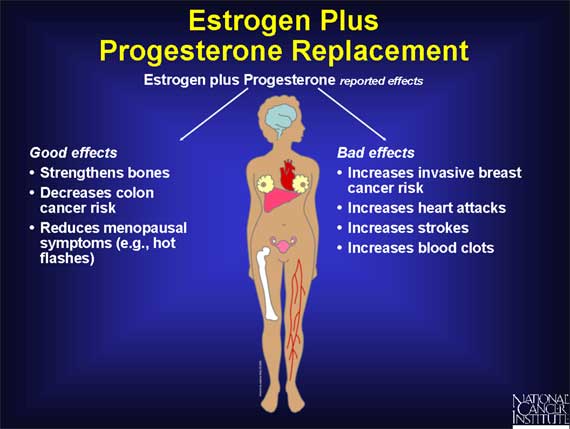
Studies carried out in the 1980s suggested that adding the hormone progesterone to estrogen can offset the increased risk of uterine cancer linked to the use of estrogen by itself. For this reason, hormone replacement therapy using estrogen plus progesterone became a common way of treating women with menopausal symptoms.
However, a study of 16,000 menopausal women carried out by the Women's Health Initiative was prematurely halted in 2002 when preliminary results indicated that the harm associated with this type of treatment outweighs the potential benefits. The major risks detected were an increased chance of developing invasive breast cancer, as well as an increased risk of strokes, heart attacks, and blood clots. While the data also revealed that hormone replacement with estrogen plus progesterone lowered the risk of osteoporosis and colon cancer, these benefits were not considered to be sufficient to outweigh the other risks.
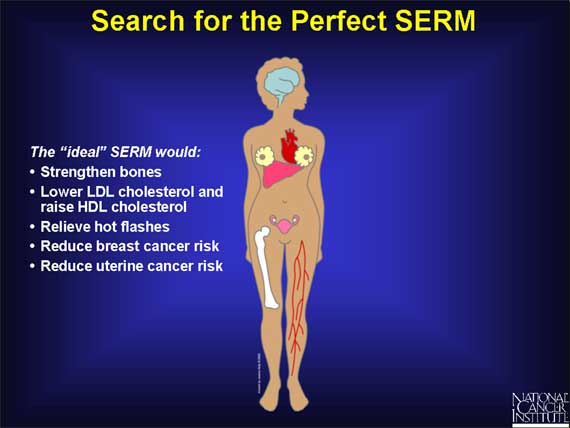
Because of the potential cancer and cardiovascular risks inherent in hormone pills containing estrogen and progesterone, scientists are working on the development of SERMs for postmenopausal women that can mimic the beneficial effects of estrogen without exerting any of its harmful effects.
The ideal drug, of course, would be a SERM exhibiting the positive effects of estrogen on bones, heart, and blood vessels, without exhibiting the potentially harmful effects of estrogen on the breast and uterus.
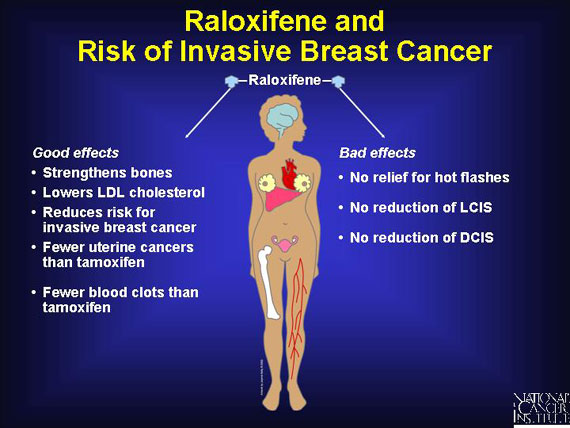
One SERM that may exhibit some of these properties is raloxifene, a drug approved by the FDA in 1997 for preventing osteoporosis in postmenopausal women.
Raloxifene appears to function like estrogen in bone, acting to maintain bone strength and increase bone density. In addition, raloxifene also resembles estrogen in its ability to lower LDL cholesterol levels, thereby decreasing the risk of heart disease.
Although information on the long-term risks and benefits of raloxifene is limited compared to tamoxifen, preliminary evidence suggests that raloxifene may exert these beneficial effects on bones, heart, and blood vessels without increasing a woman's risk of developing cancer.
In animal studies, raloxifene reduced the incidence of both breast and uterine cancer. And in preliminary human trials, raloxifene reduced the risk of breast cancer without the unwanted stimulation of uterine cell division that is exhibited by tamoxifen. As a result of these findings, the National Cancer Institute sponsored a human clinical study to directly compare the effects of tamoxifen and raloxifene in postmenopausal women at higher than average risk for this disease. The trial, named STAR (Study of Tamoxifen and Raloxifene), was begun in 1999 and is following more than 19,000 women for a period of 5 to 10 years.
Early STAR trial results show that raloxifene works as well as tamoxifen in reducing risk by about 50 percent for invasive breast cancer in postmenopausal women. And raloxifene has fewer side effects. Participants in STAR who were assigned to take raloxifene had 36 percent fewer uterine cancers and 29 percent fewer blood clots from that drug than did the women assigned to take tamoxifen.
On the other hand, tamoxifen reduces the incidence of lobular carcinoma in situ (LCIS) and ductal carcinoma in situ (DCIS) by half, while raloxifene did not have an effect on these diagnoses. (LCIS and DCIS are sometimes called noninvasive or stage 0 breast cancers.)
A woman can go to http://www.cancer.gov/bcrisktool/ to determine if her individual risk for invasive breast cancer is above average.
|