|
In animal studies, raloxifene reduced the incidence of both breast and uterine cancer. And in preliminary human trials, raloxifene reduced the risk of breast cancer without the unwanted stimulation of uterine cell division that is exhibited by tamoxifen. As a result of these findings, the National Cancer Institute sponsored a human clinical study to directly compare the effects of tamoxifen and raloxifene in postmenopausal women at higher than average risk for this disease. The trial, named STAR (Study of Tamoxifen and Raloxifene), was begun in 1999 and is following more than 19,000 women for a period of 5 to 10 years.
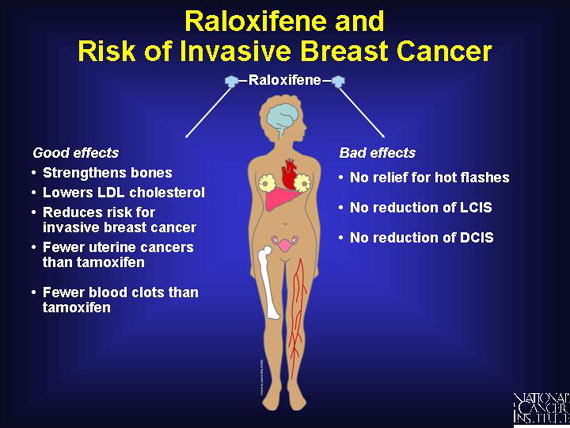
Early STAR trial results show that raloxifene works as well as tamoxifen in reducing risk by about 50 percent for invasive breast cancer in postmenopausal women. And raloxifene has fewer side effects. Participants in STAR who were assigned to take raloxifene had 36 percent fewer uterine cancers and 29 percent fewer blood clots from that drug than did the women assigned to take tamoxifen.
On the other hand, tamoxifen reduces the incidence of lobular carcinoma in situ (LCIS) and ductal carcinoma in situ (DCIS) by half, while raloxifene did not have an effect on these diagnoses. (LCIS and DCIS are sometimes called noninvasive or stage 0 breast cancers.)
A woman can go to http://www.cancer.gov/bcrisktool/ to determine if her individual risk for invasive breast cancer is above average.
< Previous | Index |