 Why does this occur? Experts in the field have looked at the reasons for these failures and have concluded that radiation therapy planning is often inadequate, providing either too little radiation to the tumor for a cure or too much radiation to nearby healthy tissue, which results in complications and sometimes death.
Why does this occur? Experts in the field have looked at the reasons for these failures and have concluded that radiation therapy planning is often inadequate, providing either too little radiation to the tumor for a cure or too much radiation to nearby healthy tissue, which results in complications and sometimes death. What can be done to improve this prognosis? Since 1993, Lawrence Livermore National Laboratory has combined its renowned expertise and decades of experience in nuclear science, radiation transport, computer science, and engineering to adapt nuclear weapons Monte Carlo techniques to a better system for radiation dose calculations. Mentored in particle interactions and nuclear data by Lawrence Livermore physicists Bill Chandler and Roger White, and armed with seed money from Livermore's Laboratory Directed Research and Development, medical physicist Christine Siantar began directing a small project to develop PEREGRINE, with the mission of providing better cancer treatment.
What can be done to improve this prognosis? Since 1993, Lawrence Livermore National Laboratory has combined its renowned expertise and decades of experience in nuclear science, radiation transport, computer science, and engineering to adapt nuclear weapons Monte Carlo techniques to a better system for radiation dose calculations. Mentored in particle interactions and nuclear data by Lawrence Livermore physicists Bill Chandler and Roger White, and armed with seed money from Livermore's Laboratory Directed Research and Development, medical physicist Christine Siantar began directing a small project to develop PEREGRINE, with the mission of providing better cancer treatment. What resulted is a radically new dose calculation system that for the first time can model the varying materials and densities in the body as well as the radiation beam delivery system. According to program manager Edward Moses, the PEREGRINE team is moving these unique radiation therapy planning capabilities into the hands of the medical community so that doctors will have the most accurate tool available to plan radiation treatments that cure cancer.
What resulted is a radically new dose calculation system that for the first time can model the varying materials and densities in the body as well as the radiation beam delivery system. According to program manager Edward Moses, the PEREGRINE team is moving these unique radiation therapy planning capabilities into the hands of the medical community so that doctors will have the most accurate tool available to plan radiation treatments that cure cancer.
PEREGRINE Breakthrough
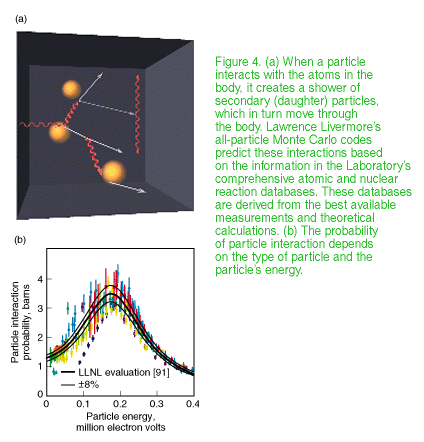
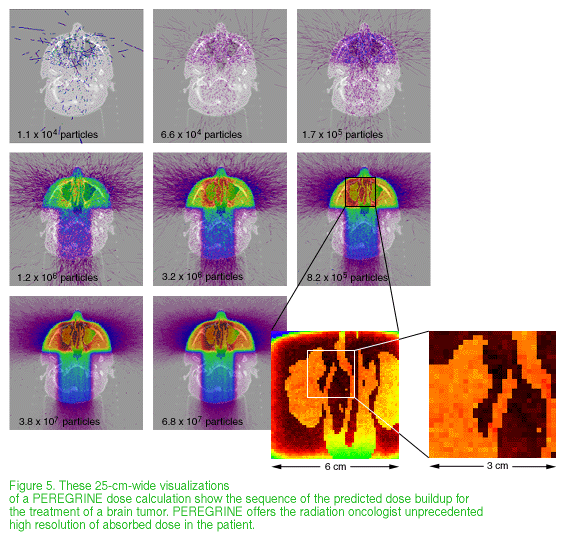
 PEREGRINE breaks the barriers to accurate dose calculation with the first full-physics model of the radiation treatment process. It uses Monte Carlo calculations, in which statistical sampling techniques are used to obtain a probabilistic approximation of a problem's solution. This enables PEREGRINE to model how trillions of radiation particles interact with the complex tissues and structures in the human body and where they deposit their energy. In the past, Monte Carlo calculations, known to be the best way to model these interactions, would have required days or weeks of supercomputer resources--impractical for radiation treatment planning. The PEREGRINE team has designed and built the Monte Carlo system to plan accurate radiation treatments at a cost and speed practical for widespread medical use. PEREGRINE uses advanced algorithms integrated with off-the-shelf computer hardware configured in sophisticated architectures to bring Monte Carlo-based treatment planning to the desktop.
PEREGRINE breaks the barriers to accurate dose calculation with the first full-physics model of the radiation treatment process. It uses Monte Carlo calculations, in which statistical sampling techniques are used to obtain a probabilistic approximation of a problem's solution. This enables PEREGRINE to model how trillions of radiation particles interact with the complex tissues and structures in the human body and where they deposit their energy. In the past, Monte Carlo calculations, known to be the best way to model these interactions, would have required days or weeks of supercomputer resources--impractical for radiation treatment planning. The PEREGRINE team has designed and built the Monte Carlo system to plan accurate radiation treatments at a cost and speed practical for widespread medical use. PEREGRINE uses advanced algorithms integrated with off-the-shelf computer hardware configured in sophisticated architectures to bring Monte Carlo-based treatment planning to the desktop.
Better Treatment Strategies
 Experts have looked at the diagnosis and treatment planning process to try to explain why current cancer treatments are not more effective.
Experts have looked at the diagnosis and treatment planning process to try to explain why current cancer treatments are not more effective.
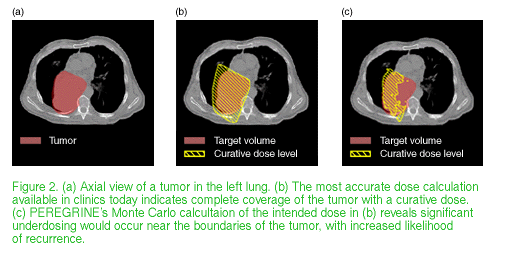
 When a physician suspects malignancy, a computerized tomography (CT) scan is made of the suspected area to determine the exact position and extent of the tumor. If the cancer has not yet metastasized and is susceptible to radiation, the next step is to develop a plan for radiation treatment (Figure 1). Although CT scans provide radiation planners with a three-dimensional (3D) electron-density map of the body, current dose calculation methods model the body as a virtually homogeneous "bucket of water." Inhomogeneities, such as bone and airways, are ignored or highly oversimplified.
When a physician suspects malignancy, a computerized tomography (CT) scan is made of the suspected area to determine the exact position and extent of the tumor. If the cancer has not yet metastasized and is susceptible to radiation, the next step is to develop a plan for radiation treatment (Figure 1). Although CT scans provide radiation planners with a three-dimensional (3D) electron-density map of the body, current dose calculation methods model the body as a virtually homogeneous "bucket of water." Inhomogeneities, such as bone and airways, are ignored or highly oversimplified.



 EDWARD MOSES, PEREGRINE program leader since 1994, received a Ph.D. (1977) in electrical engineering from Cornell University, where he specialized in quantum electronics. His earlier Lawrence Livermore experience includes program manager of the AVLIS (Atomic Vapor Laser Isotope Separation) Program from 1986 to 1990.
EDWARD MOSES, PEREGRINE program leader since 1994, received a Ph.D. (1977) in electrical engineering from Cornell University, where he specialized in quantum electronics. His earlier Lawrence Livermore experience includes program manager of the AVLIS (Atomic Vapor Laser Isotope Separation) Program from 1986 to 1990. CHRISTINE SIANTAR, principal investigator of the PEREGRINE program, received her Ph.D. (1991) in medical physics from the University of Wisconsin. Prior to joining Lawrence Livermore in 1994, she gained experience in cancer treatment planning at the Medical College of Wisconsin. Her present duties also include validation and verification of the Monte Carlo calculations for PEREGRINE.
CHRISTINE SIANTAR, principal investigator of the PEREGRINE program, received her Ph.D. (1991) in medical physics from the University of Wisconsin. Prior to joining Lawrence Livermore in 1994, she gained experience in cancer treatment planning at the Medical College of Wisconsin. Her present duties also include validation and verification of the Monte Carlo calculations for PEREGRINE.