The fact that light is a kind of wave is common knowledge
today, and a hundred years ago the theory that light is some kind of wave was
already well confirmed by experiment. But at that time Einstein found,
in a newly-discovered physical law, a clue that there was more to light than
the wave theory of those days seemed to suggest.
A B C D
A. An inconsistency
Drop a rock in a lake, and you'll stir up waves. Drop two rocks in
different places, and you'll stir up two sets of waves. If you look
closely at the two sets where they cross each other, you may find that when the
peaks of the two different waves merge, they make a higher peak; when two
troughs merge, they make a deeper trough; and when a peak from one set of waves
meets a trough from the other, the water level is intermediate.
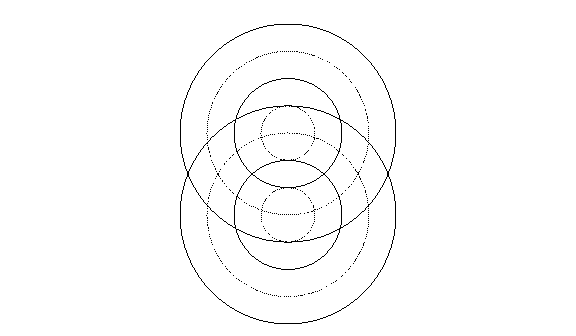
Figure 1. Schematic diagram of waves formed by rocks dropped in
a lake. Each set of concentric circles shows the arrangement of wave peaks
and troughs at one instant if just one rock were dropped in the center; solid
lines represent peaks, dotted lines troughs. If both sets of waves were
formed together, wherever peaks from both sets of waves met they would reinforce
each other; likewise, troughs from one set meeting troughs from the other set
would reinforce to make deeper troughs. Where a peak from one wave set met
a trough from the other, the water would reach an intermediate level.
Similar things happen with all kinds of waves-when waves cross, their
extremes either reinforce or cancel each other. Water waves reinforce and cancel each other's high
points and low points. Sound waves in air have alternating regions of
compressed and expanded air. When sound waves cross, at the points where
their pressure extremes meet, the extremes become more extreme as compressions
meet compressions and expansions meet expansions; while extremes become less
extreme where compressions meet expansions and the pressure averages out.
And when light waves cross, their extremes reinforce in places to make brighter
spots and cancel elsewhere to make dimmer spots. Once such bright and dim
spots in beams of light were observed in the early 19th century, physicists
became more certain that light was in fact a kind of wave, as many had suspected
since the 17th century.
New experiments and new analyses of the physical laws of light clarified the
character of light waves during the remainder of the 19th century. But at
the very end of that century, a previously obscure feature of light was
recognized, the significance of which soon caught the attention of Albert
Einstein and led to some of his most important theories relating to the behavior
of light. To better understand these theories, we first need a few details
about the earlier discoveries of others.
A few decades after bright and dim areas were discovered in light beams,
James Clerk Maxwell found a mathematical expression for the laws governing
electric and magnetic force fields. He discovered that, according to those
laws, waves could be stirred up such that the force fields would wave
perpendicularly to the waves' direction of travel, and that the waves would
travel through space at the speed of light. Since the speed and the
perpendicular waving were traits already observed in light, Maxwell proposed
that these electromagnetic waves were, in fact, light. Later, Heinrich
Hertz actually produced waves using electrical devices and showed that his waves
had all the known characteristics of light, with the exception of having much
longer wavelengths, which corresponded to the dimensions of his devices.
Electromagnetic theory that incorporated Maxwell's equations had been put to
many kinds of tests and found to be accurate as more and more experiments were
done throughout the 19th century. When Hertz discovered his
long-wavelength, low-frequency electromagnetic waves-the type now know as
radio waves-his experiments confirmed Maxwell's last major prediction about
electromagnetic force fields. But by the end of the 19th century, one
phenomenon had been discovered that didn't quite seem to fit the theory-the
distribution of energy in a particular type of light source.
Imagine a furnace whose walls reflect light and never absorb it. Inside
the furnace are some hot, glowing objects made of atoms that can produce and
absorb light waves of various frequencies. These objects might all begin
with different temperatures. But since all the light waves shining forth
from the atoms of the hot objects are reflected back by the furnace walls, every
light wave has a chance of being absorbed by one of the atoms, thus transferring
energy between the atom that produced the wave and the atom that absorbs
it. As light waves transfer energy back and forth between the atoms of the
hot objects, the temperatures of the objects tend to equalize. When this
happens, the furnace becomes a source of light waves of every possible
frequency, with the light more intense at some frequencies than at others.
It is with regard to the intensities of light from the atoms that Maxwellian
theory showed signs of trouble. In one respect, the atoms in our furnace
act like the devices Hertz used in his experiments. Hertz' devices
contained electrically charged particles that produced waves by vibrating, which
could be set to vibrating themselves when electromagnetic waves struck
them. Atoms also contain electrically charged particles. If these
charged particles behave as the ones in Hertz' apparatus were thought to behave
in his day, the intensity of light waves in a furnace should vary with the
waves' frequency in a simple way.
As it happens, though, this simple variation has little overall resemblance
to the real one. For low frequencies, the resemblance is close, but under
the assumptions made, higher-frequency waves should always be more intense than
lower frequency waves-intense enough that the total energy of all the light
waves should be infinite, which it isn't. Figure 2 below compares
the two variations; the upper curve is what the theory predicted, while the
lower curve shows how the light intensity actually does vary with frequency.
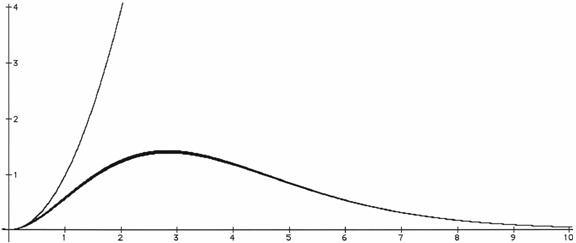
Figure
2. The upper curve indicates (incorrectly) that the intensity of light
(vertical scale) in the type of furnace described increases as the square of the
frequency (horizontal scale). This curve continues upward to
infinity. The lower curve shows the actual variation for which the light
is most intense at one particular frequency and tends toward zero for higher and
lower frequencies. The intervals between numbers on the graph depend on
the temperature; if you double the temperature, the frequency scale represents
twice the original frequency range, while the intensity scale represents 23 =
eight times the original intensity range. If the temperature is 1000
kelvins (726.85oC), each unit on the frequency scale stands for about 104
megahertz, and each unit on the intensity scale stands for roughly 167
microjoules per cubic nanometer per megahertz.
The real variation and why it is inconsistent with Maxwellian theory were
puzzles that the physicist Max Planck tried to solve for some years in in the
late 19th century. He eventually found a mathematical formula that matched
what was known from experiments. But the formula implied something that
Maxwell's theory did not.
(.....continued)
A B C
D








