 |
|
 |

Moving Cancer Stem Cells toward the Clinic
The science of cancer stem cells and how the cells might be used to prevent and detect cancer was the focus of a workshop last week on the NIH campus. The meeting was sponsored by NCI's Division of Cancer Prevention 1 (DCP).
Several hundred researchers heard presentations on what is known - and not known - about the molecular biology of cancer stem cells. Experts discussed genetic and epigenetic alterations, signaling pathways, and influence of the tumor microenvironment on cancer stem cells.
Read more 2 


 Guest Update by Dr. Henry Rodriguez
Guest Update by Dr. Henry Rodriguez
CPTI Will Help Realize the Promise of Proteomics
There are many in the cancer research community who believe proteomics has tremendous potential, particularly for the early detection of disease. Unfortunately, current proteomic methodologies have not sufficiently addressed the lack of reproducibility of measurements from run to run, instrument to instrument, or laboratory to laboratory. This lack of standards and reproducibility led NCI to develop and launch the Clinical Proteomic Technologies Initiative for Cancer 3 (CPTI) last September. The major goals of CPTI are to optimize current proteomic technologies and develop the new technologies, reagents, systems, and working teams needed to realize proteomics' promise.
Following several years of input from the international proteomics and cancer communities, CPTI was designed to provide a highly organized approach to assess, refine, develop, and apply proteomic technologies and data resources to support the discovery of biomarkers for all aspects of cancer research, especially early detection of cancer and to monitor therapeutic outcomes. This model will also lead to effective ways of addressing the barriers that exist early in the biomarker discovery pipeline.
Read more 4 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Moving Cancer Stem Cells toward the Clinic
The science of cancer stem cells and how the cells might be used to prevent and detect cancer was the focus of a workshop last week on the NIH campus. The meeting was sponsored by NCI's Division of Cancer Prevention 1 (DCP).
Several hundred researchers heard presentations on what is known - and not known - about the molecular biology of cancer stem cells. Experts discussed genetic and epigenetic alterations, signaling pathways, and influence of the tumor microenvironment on cancer stem cells.
Many researchers were enthusiastic about one day using cancer stem cells in the clinic. But they acknowledged that a lack of cancer stem cells (and patient samples) is preventing the field from exploring basic questions, such as the origins of these cells.
"The meeting reinforced the view that we need to develop technologies to isolate and characterize cancer stem cells," said Dr. Sudhir Srivastava of DCP. "Time and again people talked about having cell lines and culture systems so that more laboratories can study these cells."
Recent studies have supported a role for cancer stem cells in early detection, noted Dr. Srivastava, who heads the Cancer Biomarkers Research Group 5. But the studies have not been replicated because of a lack of adequate cancer stem cell samples from patients.
Cancer stem cells are hard to isolate because they are so rare - perhaps as few as one tumor cell in a million. The first such cells were discovered in patients with leukemia in 1997. More recently, the cells have been reported in breast, colon, and brain tumors.
Like the stem cells that repopulate adult tissues, cancer stem cells are thought to perpetuate themselves while also giving rise to diverse types of tumor cells.
In a keynote address, Dr. Michael Clarke of Stanford University said that it will be important to have diagnostic tests that can identify precancerous lesions. By the time cancer stem cells develop it is often too late to help the patient, he noted.
Dr. Clarke also stressed the critical need to understand the mechanisms by which cells escape the normal constraints on self-proliferation.
Another keynote speaker, Dr. Stewart Sell of the Ordway Research Institute, discussed how the tumor microenvironment may influence the behavior of cancer stem cells. The surrounding environment may be as important as the intrinsic properties of the cancer stem cell, he said.
He and others discussed the need to characterize genetic pathways that are deregulated in cancer stem cells, particularly those involved in self-renewal. This could lead to strategies for identifying and blocking key signals in mutant stem cells.
Cancer stem cells could also be targets for drugs to prevent cancer in patients at risk of the disease, including those with premalignant lesions, commented Dr. James Crowell, who heads NCI's Chemopreventive Agents Development Research Group 6.
The need for cancer stem cell markers was a recurrent theme. Many researchers said that multiple markers may be required to capture the genetic diversity of cancers.
Building on the success of the workshop, DCP is consulting with experts on how best to support basic research on cancer stem cells that can be translated into clinical tools.
Drs. Jacob Kagan and Levy Kopelovich of DCP, who organized the workshop, are writing a summary of the meeting and recommendations for publication.
— Edward R. Winstead
|
 |
 |

Guest Update by Dr. Henry Rodriguez
CPTI Will Help Realize the Promise of Proteomics
 There are many in the cancer research community who believe proteomics has tremendous potential, particularly for the early detection of disease. Unfortunately, current proteomic methodologies have not sufficiently addressed the lack of reproducibility of measurements from run to run, instrument to instrument, or laboratory to laboratory. This lack of standards and reproducibility led NCI to develop and launch the Clinical Proteomic Technologies Initiative for Cancer 3 (CPTI) last September. The major goals of CPTI are to optimize current proteomic technologies and develop the new technologies, reagents, systems, and working teams needed to realize proteomics' promise.
There are many in the cancer research community who believe proteomics has tremendous potential, particularly for the early detection of disease. Unfortunately, current proteomic methodologies have not sufficiently addressed the lack of reproducibility of measurements from run to run, instrument to instrument, or laboratory to laboratory. This lack of standards and reproducibility led NCI to develop and launch the Clinical Proteomic Technologies Initiative for Cancer 3 (CPTI) last September. The major goals of CPTI are to optimize current proteomic technologies and develop the new technologies, reagents, systems, and working teams needed to realize proteomics' promise.
Following several years of input from the international proteomics and cancer communities, CPTI was designed to provide a highly organized approach to assess, refine, develop, and apply proteomic technologies and data resources to support the discovery of biomarkers for all aspects of cancer research, especially early detection of cancer and to monitor therapeutic outcomes. This model will also lead to effective ways of addressing the barriers that exist early in the biomarker discovery pipeline.
Through interactions with NCI and federal and private sector groups, CPTI is expected to catalyze targeted discovery and development efforts. The purpose of these partnerships is to overcome the obstacles to recent attempts at applying protein measurement technologies - namely mass spectrometry (MS) and affinity-based detection methods - to clinical applications.
CPTI is addressing these hurdles through its three integrated programs. The team-based component is the Clinical Proteomic Technology Assessment for Cancer (CPTAC) 7, consisting of five networked technology assessment centers that conduct rigorous technology assessment, develop standard protocols and clinical reference sets, and evaluate methods to ensure data reproducibility. Centers include the Broad Institute of MIT and Harvard, Memorial Sloan-Kettering Cancer Center, Purdue University, the University of California, San Francisco, and Vanderbilt University School of Medicine. Performed in conjunction with partners such as the National Institute of Standards and Technology, CPTI has assembled a multidisciplinary team that represents a broad range of commercially available MS proteomic platforms and analysis software to provide the most comprehensive approach to assess intraplatform and interlaboratory variability to the measurement of proteins in clinical specimens.
The other components of CPTI are the principal investigator-driven Advanced Proteomic Platforms and Computational Sciences 8 and the Proteomic Reagents Resource 9. The former involves the development of innovative affinity-based and MS technologies, including software algorithms that support quantitative analysis of peptides and proteins, while the latter serves as a public resource for high-quality, well-characterized proteomic reagents (proteins, peptides, antibodies, proficiency testing materials) and other resources. Recently, CPTI's experience and the Reagents Resource component served as a model for and played a leading role in the 2008 NIH Protein Capture/Proteome Tools Roadmap Initiative.
Through its extramural community, CPTI aims to support the needed foundation for proteomics working in partnership with existing NCI resources, including caBIG, the Early Detection Research Network, and the SPOREs, to create a national proteomics research infrastructure. CPTI also integrates with NCI's technology programs such as the Innovative Molecular Analysis Technologies 10 program, Office of Cancer Genomics 11, Office of Biorepositories and Biospecimen Research 12, the Small Business Innovation Research program 13, and the NCI Alliance for Nanotechnology in Cancer 14, among others.
It's truly rewarding to be part of this effort, one that I believe can create a paradigm shift in biomarker discovery and have a significant impact on cancer detection, diagnosis, and treatment.
|
 |
 |
Common Genetic Variants Linked to Breast Cancer
Researchers have identified common genetic variations associated with breast cancer in several populations of women. The variants occur in a tumor suppressor gene called FGFR2 (Fibroblast Growth Factor Receptor 2), which was previously reported to be amplified or overexpressed in some breast cancers.
Two independent research teams - one in the U.S. and one in the U.K. - made the discovery by conducting genome-wide association studies to look for breast cancer susceptibility genes. They scanned DNA from thousands of women with breast cancer and from healthy women. In both studies, variants in FGFR2 were linked to disease risk.
"This gene is a substantial new risk factor for breast cancer," says Dr. David Hunter of the Harvard School of Public Health and NCI, and lead author of the U.S. study. "The discovery opens up new avenues for research on the gene and its signaling pathway, which may have relevance to treatment and prevention."
For women of European descent, a single copy of the variant gene increases breast cancer risk by 20 percent while two copies increase risk by 60 percent, or about the same amount as having a single family member with the disease. Approximately 15 percent of white women are estimated to carry two copies of the variant gene.
Dr. Hunter says that it would be premature to test women for the FGFR2 risk variants. Additional variants are likely to be found as the researchers analyze the genome data generated as part of the study, conducted under NCI's Cancer Genetic Markers of Susceptibility 15 (CGEMS) program.
In the study's next phase, CGEMS researchers are evaluating 29,000 variants linked to breast cancer risk during a series of collaborative studies, mostly drawn from the NCI Breast & Prostate Cancer Cohort Consortium. The aim is to build a catalogue of genetic risk factors that can be evaluated along with environmental factors, such as hormone use.
"As we sift through the genome-wide association studies, we are almost certain to find more hits that will have important implications for our understanding of breast cancer genetics," says Dr. Hunter, who is an NCI Eminent Scholar.
The findings appeared online in Nature Genetics on May 27. The same day, the U.K. group, which included researchers from NCI's Division of Cancer Epidemiology and Genetics 16 (DCEG), published findings online in Nature. Dr. Douglas Easton of Cancer Research UK at the University of Cambridge and his colleagues identified FGFR2 and three other novel breast cancer susceptibility genes (TNRC9, MAP3K1, and LSP1).
The function of these genes in breast cancer susceptibility is not known. Taken together, the new findings provide leads for identifying new biological processes in breast cancer and may contribute to the familial risk not explained by BRCA mutations.
"It's exciting and also very reassuring to see that we quickly identified a gene that was also found by another group," says Dr. Stephen Chanock, director of the NCI Core Genotyping Facility 17 in DCEG and senior author of the CGEMS study.
Dasatinib Effective Against Difficult-to-Treat ALL
The results of a 36-patient phase II clinical trial 18 indicate that the multitargeted drug dasatinib 19 (Sprycel) may be extremely beneficial in adult patients with a form of acute lymphoblastic leukemia (ALL) who have developed resistance or do not respond to another targeted agent, imatinib 20 (Gleevec). The drug's effectiveness did not appear to be hampered by most of the mutations in a key protein that have been associated with imatinib resistance.
Patients in the trial had a specific chromosomal translocation, often referred to as the Philadelphia chromosome, that is associated with a rapid course of disease after ALL diagnosis and poor survival. The translocation creates a fused protein known as BCR-ABL, the same protein typically seen in chronic myelogenous leukemia, for which imatinib has proven to be an effective treatment.
"These data are highly significant given the refractory nature of patients enrolled in this trial to current treatment modalities, including imatinib," wrote study leader Dr. Olivier Ottmann from Johann Wolfgang Goethe University in Germany and colleagues in a May 11 early online release in Blood.
In the study, patients given dasatinib at 70 mg twice daily had strong hematologic and cytogenetic response rates - meaning a return of normal white blood cell counts and a significantly reduced number of cells positive for the Philadelphia chromosome, respectively. At 8 months, 42 percent of patients had a major hematologic response, of whom two-thirds exhibited no disease progression; 58 percent of patients had complete cytogenetic responses.
Cisplatin Improves Survival for Women with Cervical Cancer
Long-term follow-up results from a Gynecologic Oncology Group clinical trial 21 that compared cisplatin-based chemotherapy with hydroxyurea in addition to radiation therapy for locally or regionally advanced cervical cancer, published online in the Journal of Clinical Oncology, showed that cisplatin-based chemotherapy significantly improved both progression-free and overall survival compared with hydroxyurea alone. The percentage of women experiencing late side effects did not significantly differ between the treatment groups.
The investigators randomly assigned participating women to one of three groups. One group received cisplatin 22 alone, the second received the chemotherapy drug hydroxyurea alone, and the third received a combination of cisplatin, hydroxyurea, and the drug 5-fluorouracil 23 (5-FU). All drugs were given during radiation therapy, and all women received the same type and amount of radiation therapy.
Patients were followed for an average of almost 9 years. Women who received either cisplatin or the combination of cisplatin, 5-FU, and hydroxyurea had significantly longer progression-free survival and overall survival than women who received hydroxyurea alone, regardless of whether the cancer had spread locally or regionally. After adjusting for the fact that more patients who received cisplatin or the combination regimen were alive for the analysis of side effects, the investigators did not observe a significant difference in late-occurring side effects between the groups.
"Collectively, this follow-up analysis continues to support the use of cisplatin-based concurrent chemotherapy with pelvic radiation therapy for locally advanced stage cervical cancer," summarized the authors.
Caspase-8 Drives TRAIL Resistance in Ewing's Sarcoma
Scientists at the Albert Ludwigs University of Freiburg in Germany and their collaborators at NCI's Center for Cancer Research 24 (CCR) have identified both a potential mechanism for resistance of Ewing's sarcoma (ES) to an experimental therapeutic protein called tumor necrosis factor apoptosis-inducing ligand (TRAIL), and a possible method for overcoming this resistance. Their results were published in the June issue of the American Journal of Pathology.
The investigators focused on caspase-8 expression because lack of the protein has been linked in laboratory studies to TRAIL resistance in ES cells and other tumors. They first looked at tissue samples taken from 47 patients with ES. In three-quarters of the samples, caspase-8 expression was detected in 60 to 100 percent of the cells. In the rest of the samples, caspase-8 expression was detected in only 0 to 50 percent of the cells. Therefore, within any individual ES tumor, cells that lack caspase-8 could cause resistance to TRAIL.
The investigators next tested whether interferon-gamma (IFN-γ), which has been shown in the laboratory to increase caspase-8 expression in cells, could sensitize ES cells with low expression of caspase-8 to treatment with TRAIL. They found that doses of IFN-γ within the range easily tolerated by patients increased caspase-8 expression in caspase-8-deficient cell lines. When the IFN-γ-treated cells were treated with TRAIL, they underwent apoptosis, indicating restored sensitivity to TRAIL.
Additional in vitro experiments showed that chemotherapy does not select for tumor cells that lack caspase-8, which would make subsequent treatment with TRAIL less effective. Changing the levels of caspase-8 expressed by the cells, either genetically or by adding IFN-γ, did not alter their sensitivity to chemotherapy, indicating "that the combination of TRAIL and IFN-γ with standard chemotherapeutics in ES could be feasible," stated the authors.
WTX Found to be a Tumor Suppressor in Wilms' Tumor
A new study has found that a protein called WTX is an important factor in the protein-destruction complex targeting β-catenin when Wnt signaling is misguided, according to results published online May 18 in Science. β-catenin activation causes Wilms' tumor.
Dr. Michael B. Major of the Howard Hughes Medical Institute and colleagues initially used proteomic methods to determine which proteins bind to β-catenin in cell lysates. They found that WTX interacts with β-catenin in the protein-destruction complex. To further investigate WTX activity, researchers generated cultured cells that express WTX. They also manipulated xenopus and zebrafish embryos to examine the effects of the Wnt gene with and without WTX.
Looking at the generated cultured cells, researchers found that WTX partners with other destruction-complex proteins to promote degradation of β-catenin. They also found that the xenopus embryos injected with the Wnt gene developed two heads, while those injected with Wnt and WTX developed milder head anomalies. They saw similar results in the zebrafish embryos. The researchers concluded that WTX negatively regulates Wnt/β-catenin signaling. Additionally, based on the ability of WTX to regulate β-catenin, it is a tumor suppressor gene in Wilms' tumor.
In an editorial, Dr. Roel Nusse of Stanford University School of Medicine noted that "β-catenin activity has been detected in other human cancers, mostly by virtue of the nuclear presence of the β-catenin protein. In many of those cases, there has been no evidence that the known components of the WNT signaling pathway are mutated, suggesting that β-catenin becomes activated without any genetic alterations. But the new knowledge by Major et al. invites speculation that WTX is in fact mutated in these cancers."
|
 |
 |

Lymphedema After Cancer - How Serious Is It?
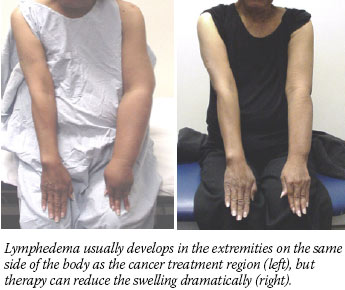 Many people who survive cancer will suffer from a serious side effect of treatment known as lymphedema, and they may not even know about it.
Many people who survive cancer will suffer from a serious side effect of treatment known as lymphedema, and they may not even know about it.
The lymphatic system comprises a very fine network of vessels and filtering nodes that circulate lymph (the fluid that bathes cells with nutrients and helps clear away waste and infection) throughout the body. Lymphedema results when part of this system is interrupted - when lymph nodes or vessels are removed or damaged, causing a "traffic jam" of lymph to build up.
Patients often describe the resulting swelling in the distal limb or tissue on the side of the body where the damage occurred as feelings of heaviness, fatigue, and tightness. Over time, swelling can increase dramatically and be accompanied by sclerosis that makes it permanent.
|
As of 2006, 21 states require insurers to cover treatment for lymphedema after cancer. To find out the requirements for insurance coverage in your state, go to NCI's State Cancer Legislative Database Program 25.
Patients can also find more information about lymphedema, including therapists and support groups in their area, by contacting the National Lymphedema Network 26.
|
Estimates for lymphedema incidence after breast cancer treatment range from 6 to 30 percent. But because the definitions of lymphedema and its severity vary, because many patients have never heard about lymphedema and so never seek medical help, and because tracking lymphedema through medical records is not required, experts agree the incidence is probably higher.
Recent research supports that contention. In the largest prospective study of its kind, published last month in Cancer Epidemiology, Biomarkers & Prevention, 32 percent of women aged 45 years and younger treated for breast cancer had persistent swelling in the hand and/or arm 3 years after surgery. The incidence of episodic swelling was 54 percent.
"This doesn't just happen in a few women," says the study's lead investigator, Dr. Electra Paskett from Ohio State University's College of Public Health and Comprehensive Cancer Center. "It's a serious consequence of treatment in terms of magnitude." Dr. Paskett is also a breast cancer survivor who lives with lymphedema.
"When I was diagnosed with breast cancer 10 years ago, during radiation I developed swelling in my affected hand and index finger," she continues. "My radiation oncologist referred me to a physical therapist, and that's how I found out about lymphedema."
Dr. Paskett's subsequent research with breast cancer survivors showed that lymphedema can have a devastating impact on quality of life, and that communication between physicians and patients on the risks for and signs of lymphedema was sparse, when it happened at all.
Why one patient may develop lymphedema while another does not is still unclear. In general, the greater the number of lymph nodes removed, the higher the risk. The risk after axillary node dissection is much greater than after sentinel node biopsy. Radiation is another risk factor, as is obesity and infection after surgery.
Everyone agrees that there is a great need for patients and physicians to understand risks for and symptoms of lymphedema, and know that there are ways to treat it and manage it.
"It's about creating pathways for the lymph fluid to drain," says Dr. Tammy Mondry, a physical therapist in San Diego, CA, who is certified by the Lymphology Association of North America 27 in lymphedema treatment. Most of Dr. Mondry's patients are breast cancer survivors, though a significant portion developed lymphedema after gynecological cancer, prostate cancer, or melanoma treatment.
Dr. Mondry performs manual lymph drainage to stimulate the pumping rate of the healthy lymph vessels throughout the body. This is one part of a four-part treatment called Complete Decongestive Therapy, which is performed 5 days a week for 2 to 4 weeks and includes compression bandaging with short stretch bandages that are worn 24 hours per day until the decrease in swelling plateaus. After that, the patient wears a compression garment daily for maintenance.
Dr. Mondry teaches people how to perform this massage on their own, as well as exercises and skin and nail care, which are especially important because the lymph that builds up in swollen limbs increases the risk for and severity of infections.
Dr. Paskett and other researchers are looking at ways to prevent lymphedema in women who receive a full axillary node dissection as part of breast cancer treatment, so that these patients can be provided with information that can keep them aware of the symptoms and allow them to obtain treatment quickly.
Awareness and diligence are crucial, because "the risk for lymphedema never goes away after surgery," explains Dr. Paskett. "We mainly see it develop in the first 12 to 18 months, but new cases continue to happen over time."
— Brittany Moya del Pino
|
 |
 |

Targeted Therapy for Lymphoid Cancers
Name of the Trial
Phase I/II Study of ABT-263 in Patients with Relapsed or Refractory T-cell or B-cell Lymphoid Malignancies (NCI-07-C-0006). See the protocol summary at http://cancer.gov/clinicaltrials/NCI-07-C-0006.
 Principal Investigator Principal Investigator
Dr. Wyndham Wilson, NCI Center for Cancer Research
Why This Trial Is Important
Lymphoid malignancies are cancers that originate in the body's lymphocytes (a type of white blood cell). A particular genetic mutation known as t(14;18) is frequently found in lymphoid cancers. This mutation causes a cell to produce too much of a protein called Bcl-2, which inhibits the process of programmed cell death (apoptosis) and contributes to tumor formation, tumor growth, and resistance to treatment.
A new drug called ABT-263 may block the activity of Bcl-2, thereby allowing cancer cells that depend on this protein for survival to undergo apoptosis. Preclinical studies have shown that ABT-263 can bind to Bcl-2 in cancer cells and prevent it from functioning, leading to cell death.
In this clinical trial, patients with T-cell or B-cell lymphoid cancers that have recurred or progressed despite prior chemotherapy will receive ABT-263 orally for up to a year. Researchers seek to establish the maximum tolerated dose of ABT-263 and to determine the drug's safety and, preliminarily, its effectiveness in these patients. They will also study ABT-263's activity in the body (pharmacokinetics).
"The Bcl-2 protein family plays a crucial role in cancer cell immortalization in B-cell lymphomas, as well as in some T-cell lymphomas and solid tumors, making it an important molecular target for these cancers," said Dr. Wilson. "This is the first study in humans of ABT-263, which was specifically designed to inhibit Bcl-2."
Who Can Join This Trial
Researchers seek to enroll 80 patients aged 18 or over with a T-cell or B-cell lymphoid cancer that has relapsed or progressed despite previous chemotherapy. See the list of eligibility criteria at http://cancer.gov/clinicaltrials/NCI-07-C-0006.
Study Site and Contact Information
This study is taking place at the NIH Clinical Center in Bethesda, MD. For more information, call the NCI Clinical Trials Referral Office at 1-888-NCI-1937. The call is toll free and confidential.
An archive of "Featured Clinical Trial" columns is available at http://cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |

NCI Participates in Two Congressional Events
NCI Director Dr. John E. Niederhuber participated with four other NIH Institute and Center directors in a Senate hearing of the Appropriations Subcommittee on Labor-HHS-Education on May 21. The theme of the hearing was "Vision of the Future - Health and Medicine (Predictive and Preemptive)." Dr. Niederhuber described the progress NCI has made in cancer research, including advances being made in gene analysis and the increasing cost effectiveness of such tests. Senators' questions for Dr. Niederhuber focused on the development of advanced technologies for early detection and treatment of disease and improving community access to care. Dr. Niederhuber's testimony highlighted some of the exciting opportunities NCI researchers are pursuing; his prepared remarks are available at http://www.cancer.gov/aboutnci/Senate-Subcommittee-on-Labor-HHS-Education-Appropriations.
On May 24, Senator Barbara Mikulski (D-MD) invited all female Senators to convene for a special public meeting on mammography, held in light of a recent NCI study 28 published in Cancer, which reported a 4 percent decrease in screening since 2000. Dr. Niederhuber and Dr. Nancy Breen of NCI's Division of Cancer Control and Population Sciences 29 were joined by a panel of experts from the Centers for Disease Control and Prevention, the American Cancer Society, and Susan G. Komen for the Cure to discuss the new statistical findings and some of the potential causes to be studied further.
 |
IOM Offers "Blueprint" for Significantly Reducing Tobacco Use
A committee convened by the Institute of Medicine has issued a new report in which it calls for major changes in how tobacco products are sold, marketed, and regulated in the United States. The report, Ending the Tobacco Problem: A Blueprint for the Nation 30, calls for FDA regulation of and increasing nationwide excise taxes on tobacco products, a nationwide nonresidential indoor smoking ban, and new restrictions on sales and marketing of tobacco products, among other actions.
"We propose aggressive steps to end the tobacco problem - that is, to reduce tobacco use so substantially that it is no longer a significant public health problem," said the committee's leader, Dr. Richard J. Bonnie, director of the University of Virginia's Institute of Law, Psychiatry, and Public Policy. The recommendations, he added, provide "a blueprint for putting the nation on a course for achieving that goal over the next two decades."
|
 |
|
|
|
 |
 |

If Memory Serves...
In the 1930s, surgery, radiation, and x-rays were the only treatments available for cancer. In its earliest years, NCI began distributing radium to about 70 hospitals throughout the country for the treatment of financially disadvantaged patients. This program lasted through the late 1960s and was favored by Congress because it brought immediate benefits to the American public. Read more 31)
For more information about the birth of NCI, go to http://www.
cancer.gov/aboutnci/ncia.
Hoover Presents NIH Gordon Epidemiology Lecture
On May 16, Dr. Robert Hoover, director of the Epidemiology and Biostatistics Program 32 in NCI's Division of Cancer Epidemiology and Genetics 16, gave the NIH Gordon Award Lecture on "Hormones and Breast Cancer: Etiology vs. Ideology." This prestigious international honor is bestowed annually on a scientist who has contributed significantly to research in epidemiology or clinical trials. The lectureship is awarded by the NIH Director on the recommendation of the NIH Epidemiology and Clinical Trials Interest Group.
Dr. Hoover reviewed the evidence for hormonally related breast cancer risk factors, some of which have run counter to prevailing hypotheses.
The lecture is named for Dr. Robert S. Gordon, Jr., the first director of the NIH Office of Disease Prevention. The event is available for viewing at http://videocast.nih.gov/.
American Society for Microbiology Honors Gottesman
The American Society for Microbiology (ASM) presented their 2007 Founders Distinguished Service Award to Dr. Susan Gottesman, head of the Biochemical Genetics Section in NCI's Center for Cancer Research 24 (CCR), for her work on behalf of ASM.
Dr. Gottesman was an editor of ASM's Journal of Bacteriology and she served as the chair of both the Division of Genetics and Molecular Biology and the Ethical Practices Committee.
ASM presented the award during their general meeting, May 21-25, in Toronto, Canada.
Teleconference Focuses on Cancer Centers Program
The final teleconference of the spring "Understanding NCI" series is scheduled for Tuesday, June 5, from 1:00-2:00 p.m., EDT. The topic is the "NCI Cancer Centers Program" with Dr. Linda Weiss, chief of NCI's Cancer Centers Branch. Within the U.S., the teleconference can be accessed toll free at 1-800-857-6584; the passcode is CENTER. Toll-free playback will be available through July 5 at 1-800-839-2204.
For additional information, go to http://ola.cancer.gov/activities/teleconferences or contact NCI's Office of Liaison Activities at 301-594-3194 or liaison@od.nci.nih.gov.
 DCCPS Report Available Online
DCCPS Report Available Online
The Division of Cancer Control and Population Sciences 29 (DCCPS) report, 2006 Overview and Highlights, was recently posted online. The report describes the division's initiatives in surveillance, molecular epidemiology, quality of care, tobacco control, behavioral research, energy balance, survivorship, health disparities, and dissemination and diffusion of new knowledge generated by cancer control research. It is available at http://cancercontrol.cancer.gov/bb/index.html.
May 31 is World No Tobacco Day
The World Health Organization has designated May 31 as World No Tobacco Day. The theme for this year's observance is smoke-free environments. More information about planned events in honor of World No Tobacco Day is available on the WHO Web site 33. The NCI Web site has more information about tobacco and cancer 34 and quitting smoking 35.
|
|
 |
 |

NCI@ASCO
Meet the Experts
Learn about NCI's program and Web sites at NCI's exhibit during the American Society of Clinical Oncology annual meeting, June 2-4.
Saturday, June 2
| 10:00 a.m. - 11:00 a.m. |
Translational Research Grant Information |
| 1:00 p.m. - 2:00 p.m. |
NCI Alliance for Nanotechnology in Cancer |
| 2:00 p.m. - 3:00 p.m. |
NCI/FDA Interagency Oncology Fellowships |
| 3:00 p.m. - 4:00 p.m. |
Technology Transfer at NIAID |
Sunday, June 3
| 11:00 a.m. - 12:00 p.m. |
Technology Transfer at NIAID |
| 1:00 p.m. - 2:00 p.m. |
NCI/FDA Interagency Oncology Fellowships |
| 3:00 p.m. - 4:00 p.m. |
New! Melanoma and Breast Cancer Risk Calculators for PDAs |
Monday, June 4
| 11:00 a.m. - 12:00 p.m. |
Cancer Survivorship Research and Care |
| 12:00 p.m. - 1:00 p.m. |
Epidemiology and Genetics Research Program Funding Opportunities |
| 1:00 p.m. - 2:00 p.m. |
NCI/FDA Interagency Oncology Fellowships |
| 2:00 p.m. - 3:00 p.m. |
Technology Transfer at NIAID |
 Featured at the NCI Exhibit
Featured at the NCI Exhibit
Get detailed information about these programs and offices at the NCI exhibit:
cancer Biomedical Informatics Grid (caBIG) 36
Center for Cancer Research 24
Division of Cancer Epidemiology and Genetics 16
Division of Cancer Prevention 37
Office of Technology and Industrial Relations 38
|
 |
 |

Metastasis Comes Into Focus at CCR
For much of the 20th century, a diagnosis of cancer terrified many who received it. Today, after some four decades of research progress in cancer detection, control, and treatment, the term that should cause more concern than "cancer" is "metastasis 39." Many oncologists agree that metastasis, not the primary tumor, is what kills most people who die from the disease.
"By the time we see patients in a clinic, the metastatic cells have often already spread," says Dr. Kent Hunter, chairperson of the Metastasis Working Group in NCI's Center for Cancer Research 24 (CCR). Unfortunately, currently available imaging technologies cannot detect very small lesions or single metastatic tumor cells. Improved patient survival after application of adjuvant therapy, however, is thought to be due to successful targeting of these subclinical micrometastases.
The metastatic cell has been called the decathlete of tumor cells, says Dr. Hunter, because it must succeed at a succession of different tasks. It has to invade the circulating blood or lymph vessels, avoid attack by the immune system, survive a lack of nutrition and cell-to-cell contact, resist shear forces as it travels, stop at a likely spot, get back into adjacent tissue, and finally establish the blood supply needed to proliferate at this new site.
Many cells begin this journey, though only a few survive it. In theory, the sequential process could be thwarted at any point. However, since tumor dissemination is often an early event, the most likely points of clinical intervention are at the end of the metastatic cascade. "You don't want to target an earlier step and find out the horse has already left the barn," says Dr. Hunter.
"Metastatic cells may be quite susceptible to new treatments early after their arrival at secondary sites and as they begin to proliferate," says Dr. Chand Khanna, head of the Tumor Metastases Section in CCR's Pediatric Oncology Branch 40. Dr. Khanna and others have been working on antimetastasis approaches based on the role of a cytoskeleton protein called ezrin that contributes to many steps in the metastatic cascade.
"Ezrin is the ERM (ezrin-radixin-moesin) protein about which we know the most," says Dr. Khanna. It sits at the cell membrane and coordinates signals about what's happening outside the cell through the interior structure (the cytoskeleton), resulting in an appropriate cellular response.
Ezrin first emerged from mouse model experiments by Dr. Khanna and Dr. Glenn Merlino, chief of CCR's Laboratory of Cancer Biology and Genetics, conducted a few years ago using a series of osteosarcoma and rhabdomyosarcoma cell lines. "When we isolated the genes involved, ezrin popped out as a player in nearly all of the cell lines that were highly metastatic, but was barely expressed in lines that were poorly metastatic." If ezrin expression proves crucial to metastasis, suppressing it should not be too difficult, and may yield a therapeutic clinical strategy.
CCR Scientific Director for Clinical Research Dr. Lee Helman became very interested because he sees many patients in the Pediatric Oncology Branch with both of these cancers. Osteosarcoma is the most common bone tumor among children - less than 20 percent of patients with metastases will be cured.
He and Dr. Khanna wondered if the ezrin expression pattern seen in the mouse models could be found in people. They obtained tumor tissue from dozens of pediatric osteosarcoma patients and the data were clear. "Ezrin was more plentiful in the patients whose disease was highly metastatic compared with those that were not. Understanding the connections and pathways that include ezrin may be a major therapeutic opportunity in a disease we really want to go after," says Dr. Helman.
Dr. Patricia Steeg, head of the Women's Cancers Section in CCR's Laboratory of Molecular Pharmacology 41, is also unraveling the mechanics of metastasis. Her discovery of Nm23, the first metastasis suppressor gene, pointed the way to a dozen others and opened up a new class of experimental models, leading to a translational strategy. "We're looking at a compound to elevate Nm23 in micrometastatic tumor cells," she explains, with the hope of interfering with colonization in high-risk cancer patients.
— Addison Greenwood
|
 |
|
Table of Links
| 1 | http://prevention.cancer.gov |
| 2 | http://cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_052907/page2 |
| 3 | http://proteomics.cancer.gov |
| 4 | http://cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_052907/page3 |
| 5 | http://prevention.cancer.gov/programs-resources/groups/cb |
| 6 | http://prevention.cancer.gov/programs-resources/groups/cad |
| 7 | http://proteomics.cancer.gov/programs/CPTAC |
| 8 | http://proteomics.cancer.gov/programs/platforms |
| 9 | http://proteomics.cancer.gov/programs/reagents_resource |
| 10 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_091206/page3 |
| 11 | http://ocg.cancer.gov |
| 12 | http://biospecimens.cancer.gov |
| 13 | http://sbir.cancer.gov |
| 14 | http://nano.cancer.gov |
| 15 | http://cgems.cancer.gov/index.asp |
| 16 | http://dceg.cancer.gov |
| 17 | http://cgf.nci.nih.gov/home.cfm?CFID=1707295&CFTOKEN=92338939 |
| 18 | http://www.cancer.gov/clinicaltrials/UCLA-0411071-01 |
| 19 | http://www.cancer.gov/cancertopics/druginfo/dasatinib |
| 20 | http://www.cancer.gov/cancertopics/druginfo/imatinibmesylate |
| 21 | http://www.cancer.gov/clinicaltrials/GOG-120 |
| 22 | http://www.cancer.gov/cancertopics/druginfo/cisplatin |
| 23 | http://www.cancer.gov/cancertopics/druginfo/fluorouracil |
| 24 | http://ccr.nci.nih.gov |
| 25 | http://www.scld-nci.net |
| 26 | http://www.lymphnet.org |
| 27 | http://www.clt-lana.org |
| 28 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_051507/page4 |
| 29 | http://dccps.nci.nih.gov |
| 30 | http://books.nap.edu/catalog.php?record_id=11795#toc |
| 31 | http://cancer.gov/aboutnci/if-memory-serves |
| 32 | http://dceg.cancer.gov/epbiostat-intro.html |
| 33 | http://www.who.int/en |
| 34 | http://www.cancer.gov/cancertopics/smoking |
| 35 | http://smokefree.gov |
| 36 | http://cabig.cancer.gov/index.asp |
| 37 | http://www.cancer.gov/prevention |
| 38 | http://otir.cancer.gov |
| 39 | http://www.cancer.gov/cancertopics/factsheet/Sites-Types/metastatic |
| 40 | http://home.ccr.cancer.gov/oncology/pediatric |
| 41 | http://ccr.cancer.gov/labs/lab.asp?labid=102 |
|
 |