CBER Presentation
Printable Version
Note: Documents in PDF format require the Adobe Acrobat Reader®. If you experience problems with PDF documents, please download the latest version of the Reader®.
Cellular, Tissue and Gene Therapies: Office Update
6th Annual Somatic Cell Therapy Symposium
September 25, 2006
Celia M.Witten, Ph.D., M.D.
Director, Office of Cellular, Tissue, and Gene Therapies
Outline
- Organizational Structure
- Guidance/Regulation Update
- Pandemic Flu Planning
- Advisory Committee Meetings
- Research Update
- Liaison Meetings/Activities
- Standards Activities
- Other outreach/collaborations
- RSVP Program
Office of Cellular, Tissue, and Gene Therapies
Celia M.Witten, Ph.D, M.D.
Recruiting, Office Deputy Director
Richard McFarland, Ph.D, M.D. Associate Director for Policy
Suzanne Epstein, Ph.D., Associate Director for Research
Deborah Lavoie, J.D. Director RPM
Division of Cellular and Gene Therapies
Raj Puri, Ph.D., M.D., Director
Division of Human Tissues
Ruth Solomon, M.D., Director
Division of Clinical Evaluation and Pharmacology/Toxicology
Ashok Batra, M.D.
Guidance/Regulation Update
- Draft Guidance: Gene Therapy Clinical Trials
- Observing Participants for Delayed Adverse Events
- Guidance for Industry: MedWatch Form FDA 3500A: Mandatory Reporting of Adverse Reactions Related to Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/P's)
- References for the Regulatory Process for the Office of Cellular, Tissue and Gene Therapies (OCTGT)" that we posted at the following URL-- http://www.fda.gov/cber/genadmin/octgtprocess.htm
- Chronic Cutaneous Ulcer and Burn Wounds-Developing Products for Treatment http://www.fda.gov/cber/gdlns/ulcburn.htm
Annual Guidance Document Agenda: OCTGT
- Licensure of Minimally Manipulated, Unrelated, Allogeneic Placenta/Umbilical Cord Blood Intended For Hematopoietic Reconstitution in Patients With Hematological Malignancies
- Preparation of Investigational Device Exemptions and Investigational New Drugs for Products Intended to Repair or Replace knee Articular Cartilage
- Initiation and Conduct of Clinical Trials Using Cellular Therapies for Cardiac Disease
- Potency Measurements for Cell and Gene Therapy Products
- Considerations for Allogeneic Pancreatic Islet Cell Products
- Current Good Tissue Practice for Human Cell, Tissue, and Cellular and Tissue Based Product Establishments
- Certain Distributed and Inventoried Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) Recovered From Donors Who Were Improperly Tested
- Clinical Study Design for Early Phase Studies of Cellular and Gene Therapies
- Devices Involved in Manufacture, Storage and Administration of Cellular Products and Tissues
- Validation of Rapid Microbiological Methods for Assessing Sterility of Cellular and Gene Therapy Products
- Submission of Information for the National Xenotransplantation Database
- Registration and Listing for Human Cell, Tissue, and Cellular and Tissue-Based Products Establishments
- Preparation of Investigational Device Exemptions and Investigational New Drugs for Tissue Engineered and Regenerative Medicine Products
- Facilities and Controls for Cellular and Gene Therapy Products Manufacturing Operations Guidance
- Link for Docket number: 2004N-0234 http://www.accessdata.fda.gov/scripts/oc/ohrms/frbydocket.cfm
Pandemic Flu Planning
- FDA is developing a comprehensive agency-wide pandemic influenza preparedness plan
- Each CBER office is developing a detailed strategic plan to be incorporated into a Center plan
- Ensure that critical activities will continue in event of a pandemic
- Ensure that critical communication with industry is maintained
- Address approach to product availability issues
- CBER Medical Director for Emerging and Pandemic Threat Preparedness: Dr.Mark Goldberger
Advisory Committee Meetings (CTGTAC)
CTGTAC Meeting (9/29/05)
- Office-wide site visit to review OCTGT research
- CTGTAC members and invited experts
- Critical path needs, priorities
- Management practices
- Examples of accomplishments
CTGTAC 2/9/06-2/10/06
- Potency measurements for cellular and gene transfer products
- National Toxicology Program on Retroviral Mutagenesis
- Review of Office Site Visit
Potency Assay Development
- Approaches to assay development
- Biological assays
- Analytical assays
- Challenges to assay development
- Product characteristics
- Assay characteristics
- "Matrix" approach
NTP Collaborative Study
- National Toxicology Program, Collaborative Study: FDA, NIH, Chris Baum, David Emery
- Systematic approach to study effect of various parameters on risk of vector-mediated tumorigenesis:
- Delete U3 from LTR (SIN)
- Insert Insulator element in LTR
- Modulate MOI used for vector transduction
- Include both gammaretrovirus and lentivirus vectors
- Mouse model established by Chris Baum
- Analyze in study of sufficient size to give 90% CI in negative result
Science in Research and Review
- Bring scientific advances to medical product development process (simulation models, validated biomarkers, new clinical trial designs)
- Stimulate development of applied research programs in critical path scientific areas, aim to develop techniques that address challenges encountered during product development
- Regulatory guidance/practice and standards to reflect best available science, integrate FDA involvement
Research Program Areas
- Virology
- Retroviruses, adeno, herpes, PERV
- Immunology
- Host-vector interactions, transplant rejection
- Cell biology
- Control of differentiation in animal models, stem cell biology
- Cancer biology
- Molecular biomarkers, animal models
- Biotechnology
- Microarray, flow cytometry
Research Examples: Virology
- When adenovirus vectors are injected intravenously, they rapidly target and kill Kupffer cells in the liver.
- This mechanism contributes to the hepatotoxicity of adenovirus vectors.
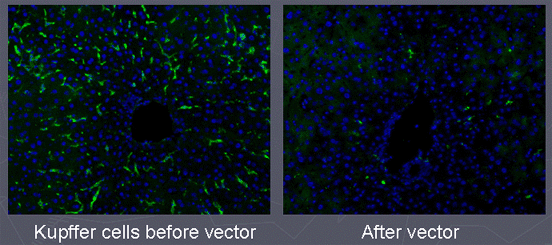
Manickan, E., J.S. Smith, J. Tian, J.N. Lozier, T.L. Eggerman, J. Muller and A.P. Byrnes (2006). Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Molecular Therapy 13: 108-117
Research Example: Virology
- Records from Cleveland Family Study, conducted before and during the 1957 pandemic (shift from subtype H1N1 to H2N2), were analyzed.
- Adults who had symptomatic influenza A in earlier study years were less likely to have it during the pandemic. Children did not show this apparent protective effect.
- These findings suggest an impact of accumulated heterosubtypic immunity during a pandemic.
- Epstein, S.L. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: An experiment of nature. Journal of Infectious Diseases, 193:49-53, 2006.
Research Examples: Immunology
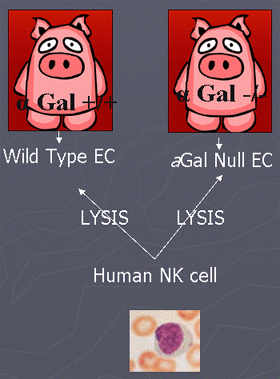
- Gala (1,3)-Gal (aGal) is the main antigen in human antibody-mediated recognition of porcine endothelial cells (EC) in hyperacute rejection
- However, NK cells recognize both aGal +/+ and aGal -/- cells equivalently
- Elimination of aGal alone from source pigs will be insufficient to circumvent the NK cell mediated destruction of porcine EC.
- Horvath-Arcidiacono, J.A., Porter, C. M., Bloom, E. T. Human NK cells can lyse porcine endothelial cells independent of their expression of Gala (1,3)-Gal (aGal) and killing is enhanced by activation of either effector or target cells. Xenotransplantation, 13:138-327, 2006.
Research Example: Biotechnology
- Microarray profiling was used to identify cluster of genes that correlate with overconfluent state of 293 cell substrate and adnenoviral vector yield
- A cluster of genes in overconfleunt state correlated with poor yield of viral vector
- Gene expression profiling may be a new tool to assess the quality of cell substrates prior to large-scale production of a biological product (e.g., viral vectors, recombinant proteins, or vaccines)
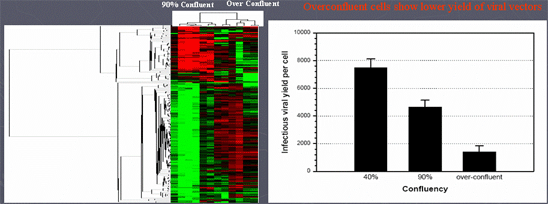
Han, J., Farnsworth, R.L, Tiwari, J.L, Tian, J., Lee, H. Ikonomi. P., Byrnes, A.P., Goodman, J.L., Puri, R. K. Quality Prediction of Cell Substrate Using Gene Expression Profiling. Genomics 87, 552-559, 2006. doi:10.1016/j.ygeno.2005.11.017.
Liaison Meetings/Liaison Activities
- ISCT
- TERMC (NRMF/PTEI)
- AABB
- AAOS
- AATB
- EBAA
- ASRM
Standards Activities
- ASTM
- Committee F04.04 on Division lV - Tissue Engineered Medical Products
- OCTGT votes on subcommittee ballots
- Input on FDA ballot on committee ballots
- ASTM F04, Division IV, Toronto, May 16-19, 2006 participation
- Strategic Planning for Division IV
- Classification and Terminology, Biomaterials and Biomolecules, Cells and Tissue Engineered Constructs, Assessment, Adventitious Agents Safety
- Organizational Meeting for Cell Signaling Subcommittee
- Next meeting November 2006, Atlanta
- ISO
- OCTGT input through FDA representative
- ISO/TC 194/SC 1 on TSE elimination
Outreach/Collaborations
- Professional society presentations
- ICH (inadvertent germline transmission, oncolytic virus, pharmacogenomics, pharm/tox)
- WHO (tissues, xeno.)
- ICCVAM
- IOTF
- MATES
Regulatory Site Visit Training Program
- Opportunity to observe development and manufacturing practices
- Enhance understanding of current practices, regulatory impacts and needs
- Enhance communication
- http://www.fda.gov/cber/regsopp/8115.htm
Contact Information
Celia Witten, PH.D., M.D.
Office Director, OCTGT
CBER/FDA
1401 Rockville Pike (HFM 70)
Rockville, MD 20852-1448
celia.witten@fda.hhs.gov
301-827-5102


