Product Approval Information
Statistical Review and Evaluation
| BLA#: | 125126/419 |
| Product: | GARDASIL: Human Papillomavirus [Types 6, 11, 16 and 18] Recombinant Vaccine – Expanded Efficacy |
| Indication: | Prevention of vulvar and vaginal disease and cancer caused by HPV types targeted by the vaccine. |
| Applicant: | Merck & Co, Inc. |
| Date: | September 18, 2008 |
| From: | Martha Lee, Ph.D., HFM-217 |
| Through: | A. Dale Horne, Branch Chief, VEB |
| To: | Nancy Miller, HFM-437 Elizabeth Valenti , HFM-481 |
| CC: | Steve Anderson, HFM-210 Henry Hsu, HFM-215 A. Dale Horne, HFM-217 Robert Ball, HFM-222 Chron, DCC |
TABLE OF CONTENTS
II.3 Clinical Efficacy Endpoints
II.5 Efficacy Results of Clinical Studies
V. COMMENTS AND QUESTIONS TO CBER REVIEW COMMITTEE
VI. COMMENTS AND QUESTIONS TO APPLICANT
LIST OF TABLES
Table 1. Clinical Efficacy Studies of GARDASIL
Table 2. Definition of Analysis Populations
Table 10. Analysis of Efficacy Against HPV 16/18-Related VIN 2/3 or VaIN 2/3 or Worse
Table 17. Analysis of Efficacy Against HPV 6/11-Related Condyloma (Protocols 013 and 015 Combined)
GARDASIL is a non-infectious recombinant, quadrivalent vaccine prepared from the highly purified virus-like particles (VLPs) of the major capsid (L1) protein of HPV Types 6, 11, 16, and 18. It is indicated in girls and women 9 through 26 years of age. The original license application for GARDASIL was approved in 2006 on the basis that GARDASIL demonstrated high efficacy in preventing cervical cancer caused by vaccine HPV types in pre-specified interim analysis of pivotal efficacy studies.
On March 30, 2007, Merck submitted a supplemental BLA (125126/419) in which the cross protection data was included. As a result of the May 30, 2007 teleconference between CBER and Merck, Merck separated the supplement originally filed on March 30, 2007 into two supplements (STN 125126/419 and STN 125126/----).
This supplemental application (STN 125126/419) is to extend the original indication to include prevention of vulvar and vaginal disease and cancer caused by HPV types targeted by the vaccine.
This statistical review focuses on clinical efficacy for HPV 16 and HPV 18-related grade 2 and grade 3 vulvar intraepithelial neoplasia (VIN) and grade 2 and grade 3 vaginal intraepithelial neoplasia (VaIN) that are precursors of vulvar and vaginal cancer .
II. CLINICAL STUDIES
Three clinical efficacy studies of GARDASIL (Protocols 007, 013, and 015) provide data in the current supplemental application. Table 1 presents brief description of these 3 studies.
Study design, efficacy endpoints, statistical methodology, and study results will be included in the following subsections.
Table 1 . Clinical Efficacy Studies of GARDASIL
Study |
Phase |
Gender: Number of randomized subjects Mean Age Age Range at Enrollment |
External Genital and Vaginal Disease Efficacy Demonstration |
|---|---|---|---|
007 |
IIb |
Part B Female: 551 20.0 years 16 to 23 years
|
To demonstrate: Efficacy: (1) Administration of a 3-dose regimen of GARDASIL™ to 16- to 23-year old women is highly effective in preventing acquisition of HPV 6, 11, 16, or 18 related vaginal and vulvar disease or cancer through 5 years of follow-up. Immunogenicity : (1) Administration of a 3-dose regimen of GARDASIL to 16- to 23-year-old women induces anti- HPV responses that can be detected in most subjects through 5 years of follow-up; (2) Administration of GARDASIL induces strong immune memory responses; and (3) Administration of a fourth dose of GARDASIL™ 4.5 years following completion of the primary vaccination series results in strong anamnestic responses. |
013 |
III |
Female: 5455 20.3 years 16 to 24 years |
To demonstrate that administration of a 3-dose regimen of GARDASIL™ reduces the incidence of the composite endpoint of HPV 6-, HPV 11-, HPV 16-, or HPV 18-related external genital lesions (EGL) (including condyloma acuminata, vulvar intraepithelial neoplasia [VIN] of any grade, and vaginal intraepithelial neoplasia [VaIN] of any grade) |
015 |
III |
Female: 12,167 19.9 years 15 to 26 years |
To demonstrate: (1) Prophylactic administration of GARDASIL™ to 16- to 26-year-old women is efficacious in preventing the development of HPV 16- and HPV 18-related VIN 2/3 or VaIN 2/3, thereby preventing the development of HPV 16- and HPV 18-related vulvar and vaginal cancer, respectively; (2) Prophylactic administration of GARDASIL to 16- to 26-year-old women is efficacious in preventing the development of HPV 6-, HPV 11-, HPV 16-, and HPV 18-related EGL. Evidence of protection against these endpoints appears during the period in which the 3-dose regimen is being administered; |
Phase IIb Efficacy Study (Protocol 007)
Protocol 007 was a randomized, double-blind, placebo-controlled, multicenter study. Subjects were randomized to 1 of 3 formulations of Quadrivalent HPV (Types 6, 11, 16, 18) L1 VLP vaccine (20-/40-/40-/20-mcg, 40-/40-/40-/40-mcg, or 80-/80-/40-/80-mcg) or placebo. Each formulation was administered at 0, 2, and 6 months. Under the original protocol, subjects were followed for a total of 3 years. Pelvic examinations were conducted at Day 1 and at Months 7, 12, 18, 24, 30, and 36 for Pap testing and to obtain cervicovaginal/genital swabs for HPV PCR. Serum samples were collected at Day 1 and Months 2, 3, 6, 7, 12, 18, 24, 30, and 36 for the assessment of baseline HPV serostatus, anti-HPV responses to vaccination, and the persistence of such responses. External genital warts or lesions noted any time during the study were to be biopsied. Guidelines were given for triage of abnormal Pap tests to colposcopy. Cervical biopsy specimens were collected if medically indicated. Biopsy samples were read by an independent Pathology Panel for assignment of endpoint status.
Of the 1,158 subjects who enrolled in Protocol 007, 551 received either GARDASIL (276 subjects) or placebo (275 subjects) in the dose-ranging phase of the study (“Part B”). Data based on this study cohort are included in this submission.
Phase III Efficacy Studies (Protocols 013 and 015)
Protocol 013 was a randomized, double-blind, placebo-controlled, multicenter efficacy study consisting of 2 immunogenicity substudies. About 5400 subjects were randomized in the 2 substudies, which were designed to assess the quadrivalent HPV vaccine’s impact on HPV 6/11/16/18-related clinical disease. The Study (Protocol 013) had 2 treatment groups: group that received GARDASIL (n=2700) and HPV vaccine placebo group (n=2700). The efficacy study (Protocol 013) was conducted by randomizing subjects into each treatment group in the context of 2 separate immunogenicity substudies (a concomitant administration substudy which included the administration of hepatitis B vaccine (Recombinant) (Protocol 011) and a monovalent HPV 16 bridging substudy (Protocol 012).
Protocol 015 was a prospective randomized, double-blind, placebo-controlled, parallel, multicenter study. Embedded within Protocol 015 is a consistency lot substudy. Approximately 12,100 subjects were randomized in a 1:1 ratio to receive either 3 injections of quadrivalent HPV (Type 6, 11, 16, 18) L1 VLP vaccine or 3 injections of placebo. Within the quadrivalent HPV vaccine group, approximately 1500 of the subjects were further randomized to receive 3 different lots of the vaccine in a 1:1:1 ratio. The actual lots received were expected to differ between subjects enrolled in the consistency lot substudy and the remaining subjects in Protocol 015. Approximately 1 month following the third vaccination visit (Month 7), 6 months following the third vaccination visit (Month 12), and every year thereafter until Month 48, subjects were to return to the study center for collection of specimens, gynecologic examinations, and Pap tests.
II.3 Clinical Efficacy Endpoints
This review gives attention to the vaccine HPV type-specific vulvar and vaginal disease endpoints.
Protocol 007 The composite disease endpoint included genital warts (condyloma acuminata), VIN, VaIN, Vulvar Cancer or Vaginal Cancer related to HPV 6, 11, 16, or 18. A subject was considered to have a case of genital warts, VIN, VaIN, Vulvar Cancer or Vaginal Cancer related to HPV 6, 11, 16, or 18 if she had both of the following on an individual biopsy:
- a consensus Pathology Panel diagnosis of genital warts, VIN 1, VIN 2, VIN 3, VaIN 1, VaIN 2, VaIN 3, Vulvar Cancer, or Vaginal Cancer; AND
- detection of HPV 6, 11, 16, or 18 by Thinsection PCR in an adjacent section from the same tissue block.
Thinsection PCR was introduced into Protocol 007 after study start. Thus, if Thinsection
PCR results were not available for an individual biopsy, it could be counted as an efficacy endpoint if:
- the biopsy had a consensus Pathology Panel diagnosis of genital warts, VIN 1, VIN 2, VIN 3, VaIN 1, VaIN 2, VaIN 3, Vulvar Cancer, or Vaginal Cancer;
- HPV 6, 11, 16, or 18 was detected by PCR in tissue collected from an adjacent biopsy for PCR testing; AND (3) the same HPV type was detected in a cervicovaginal/external genital swab collected at the routine visit immediately preceding or following the date of the biopsy.
If neither Thinsection PCR result nor PCR result on frozen adjacent biopsy tissue was available, then an endpoint could occur if on an individual biopsy:
- the biopsy had a consensus Pathology Panel diagnosis of genital warts, VIN 1, VIN 2, VIN 3, VaIN 1, VaIN 2, VaIN 3, Vulvar Cancer, or Vaginal Cancer; AND
- HPV 6, 11, 16, or 18 was detected by PCR on a swab collected from the biopsy site; AND
- the same HPV type was detected in a cervicovaginal/external genital swab collected at the routine visit that immediately preceded or followed the date of the biopsy.
Protocol 013 and Protocol 015 A co-primary endpoint of Protocol 013 and a secondary endpoint of Protocol 015 was the combined incidence of genital warts (condyloma acuminata), VIN, VaIN, Vulvar Cancer or Vaginal Cancer related to HPV 6, 11, 16, or 18. A subject was considered to have a case of genital warts, VIN, VaIN, Vulvar Cancer or Vaginal Cancer related to HPV 6, 11, 16, or 18 if she had both of the following on an individual biopsy:
- a consensus Pathology Panel diagnosis of genital warts, VIN 1, VIN 2, VIN 3, VaIN 1, VaIN 2, VaIN 3, Vulvar Cancer, or Vaginal Cancer; AND
- HPV 6, 11, 16, or 18 detected by Thinsection PCR in an adjacent section from the same tissue block.
Analysis Populations
All populations for the analysis of prophylactic efficacy are defined in Table 2. The per-protocol population (PPE) was the main analysis population. Other populations were used for supporting purposes.
Table 2 . Definition of Analysis Populations
|
PPE |
MITT-2 |
MITT-3 |
RMITT-2 |
|---|---|---|---|---|
Definition |
A vaccine HPV type-specific population:
-Naïve by serology and PCR to the relevant HPV type at Day 1 -Free of infection with the relevant HPV type through Month 7 -Received all 3 doses of study vaccine No major protocol violations |
An HPV type-specific population:
-Naïve by serology and PCR to the relevant vaccine HPV type at Day 1 - Naïve by PCR to at least one of 10 common high-risk non-vaccine HPV types at Day 1 (where relevant) - Negative Pap Test at Day 1 (where relevant) - Received at least 1 dose of study vaccine |
General study population:
- Regardless of initial serology and PCR status - Received at least 1 dose of study Vaccine
|
A single population:
- Naïve by serology and PCR to all vaccine HPV type at Day 1 -Naïve by PCR all 10 common high-risk non-vaccine HPV types at Day 1 - Negative Pap Test at Day 1 - Received at least 1 dose of study vaccine |
Case Counting |
Starting after the Month 7 Visit |
Starting after Day 30 |
Starting after Day 30 |
Starting after Day 30 |
Statistical methods
For different analysis populations, cases were counted beginning at different time points (visits) as indicated in the population definitions. In general, each subject’s follow-up time was computed by calculating the number of days between the visit date at which case counting started and her final visit date and converting this value to person-years by dividing by 365.25. For subjects who became cases, the final visit date was the visit date at which the endpoint of interest was detected (for infection, this was the first of the 2 consecutive PCR-positive visits). If a subject developed more than one case of a given endpoint, the final visit date was the date at which the first case of the endpoint was detected. For subjects who were non-cases for cervical endpoints, the final visit date was the date representing the last opportunity to observe a cervical endpoint, defined as the latest of the subject’s cervical specimens (biopsies, ECCs, definitive therapy, or Pap test). All incidence rates provided were person-time-based.
Vaccine efficacy (defined as [1 – Relative Risk]*100%) and the corresponding 95% confidence intervals were estimated using an exact procedure which accounted for the amount of follow-up (i.e., person-time at risk) in the vaccine and placebo groups. Subjects were pooled across the studies by vaccination group (vaccine or placebo) for analysis.
Cumulative incidence distribution functions (defined as 1 minus the survival distribution function) presented graphically were obtained using the nonparametric product-limit method of computing the survival distribution function The graphs are truncated at Month 36, due to the small proportion of subjects who had follow-up visits beyond Month 36 as of the cutoff date.
Estimation of Vaccine Efficacy by Categories of a Baseline Covariate: Estimates of vaccine efficacy within categories of baseline covariates were obtained within each category of an index baseline covariate by pooling data from Protocols 007, 013, and 015 using an exact procedure.
The sparseness of endpoint cases in the group vaccinated with GARDASIL in the PPE population precluded the assessment of baseline covariate-adjusted efficacy using statistical models.
Cox’s proportional hazards regression models were used in assessments of association between baseline covariates and the risk of becoming an endpoint case in the PPE Placebo group only due to the sparseness of endpoint cases in the group vaccinated with GARDASIL in the PPE population.
II.5 Efficacy Results of Clinical Studies
On February 6, 2008 (STN 125126 Supplement -------), the applicant submitted study reports of Protocol 013 and Protocol 015 based on the study completion data. Additional tables were included in STN 125126 Supplement ------- (February 22, 2008). These reports provide information of study results for this review.
Results of Individual Studies
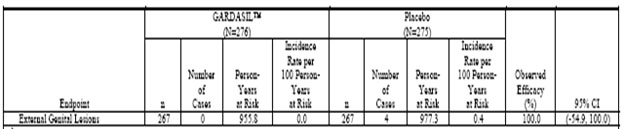
Phase IIb Efficacy Study (Protocol 007). The estimates of vaccine efficacy included in Table 3, Table 4, and Table 5 (Tables 4-3 (Cont.), 4-4 (Cont.), and 4-5 (Cont.) in Reference 2047, respectively) are based on the combined data from 3 years of follow-up Postdose 1 for subjects who did not participate in the extension phase and 5 years of follow-up Postdose 1 for those who did.
Table 3 . Analysis of Efficacy Against HPV 6/11/16/18-Related Persistent Infection or Disease From 30 Days after Day 1 Through Month 60 (All Subjects Through Month 36 and Extension Subjects Through Month 60) (Modified-Intention-to-Treat 2 Efficacy Population)
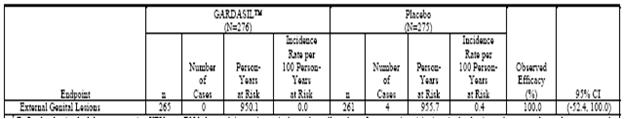
Table 4 . Analysis of Efficacy Against HPV 6/11/16/18-Related Persistent Infection or Disease From 30 Days After Day 1 Through Month 60 (All Subjects Through Month 36 and Extension Subjects Through Month 60) (Modified-Intention-to-Treat 3 Efficacy Population)
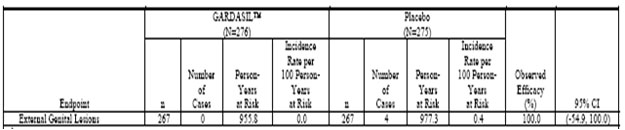
Table 5 . Analysis of Efficacy Against HPV 6/11/16/18-Related Persistent Infection or Disease From 30 Days After Day 1 Through Month 60 (All Subjects Through Month 36 and Extension Subjects Through Month 60) (Modified-Intention-to-Treat 3 Efficacy Population)
Reviewer’s Comments: The primary objective of Protocol 007 was to select a dose of quadrivalent HPV (Types 6, 11, 16, 18) L1 virus-like particle (VLP) vaccine for use in Phase III clinical trials. The Phase III dose was selected in Jun- 2001 based on the interim analysis of immunogenicity. A secondary objective of the study was to evaluate the efficacy of GARDASIL against the composite endpoint of vaccine-type HPV-related persistent infection or disease, compared with placebo. In other words, VIN 2/3 and VaIN 2/3 were not separated out as individual endpoints; they were only two components of the composite endpoint. Hence, results from Protocol 007 are not included in evaluating efficacy of GARDASIL against these diseases in this review.
Phase III Efficacy Study (Protocol 013). Table 6 (Table 11-10 in Reference P013) presents the primary assessment of efficacy of GARDASIL in preventing HPV 6-, HPV 11-, HPV 16-, or HPV 18-related external genital lesions (EGLs) in the PPE population, by HPV type and by lesion type. No multiplicity adjustment was made for HPV type-specific analyses of efficacy. Most EGLs were HPV 6-, 11-, or 16-related. The majority of lesions were low-grade (condyloma, VIN1, or VaIN 1). No cases of vulvar or vaginal cancer were observed.
In particular, high efficacy of VIN 2/3 and VaIN 2/3 was observed; the lower bound of the 95% confidence interval for VIN 2/3 or VaIN 2/3 caused by vaccine HPV types was 67.0%.
Table 6 . Analysis of Efficacy Against HPV6/11/16/18-Related External Genital Lesions (Per-Protocol Efficacy Population)
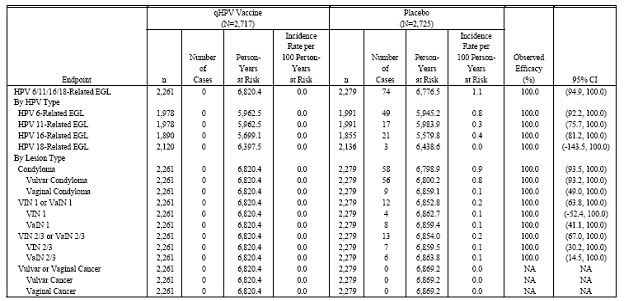
Table 7and Table 8(Table 11-11 and Table 11-12 in Reference P013) summarize the results of the efficacy analyses with respect to HPV 6-, HPV 11-, HPV 16-, or HPV 18-related EGLs, overall, by HPV type, and by lesion type, in the MITT-2 and the MITT-3 population, respectively.
Table 7 . Analysis of Efficacy Against HPV 6/11/16/18-Related External Genital Lesions (Modified Intention-to-Treat Population 2)

Table 8 . Analysis of Efficacy Against HPV 6/11/16/18-Related External Genital Lesions (Modified Intention-to-Treat Population 3)
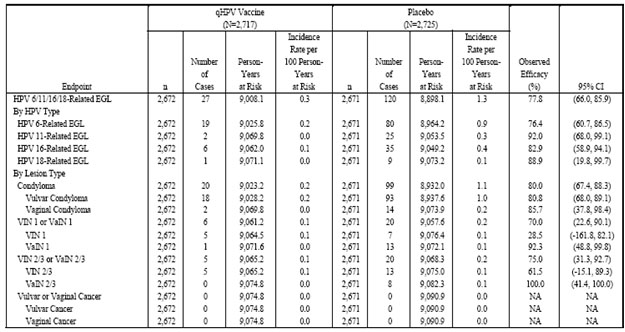
Phase III Efficacy Study (Protocol 015). The analyses provided represent the entire study follow-up period, from enrollment of the first study subject at the first site, to the final efficacy follow-up visits in all subjects.
Table 9 (Table 11-9 in Reference P15) presents the analysis of the impact of administration of GARDASIL on the rates of HPV 6-, HPV 11-, HPV 16-, or HPV 18-related EGLs in the PPE population. Analyses of the impact of GARDASIL on the combined incidence of HPV 16- and HPV 18-related VIN 2/3 or VaIN 2/3 are presented in Table 10 (Table 11-10 in Reference P15). No cases of vulvar or vaginal cancer were observed in the study.
Table 9. Analysis of Efficacy Against HPV 6/11/16/18-Related External Genital Lesions (Per-Protocol Efficacy Population)
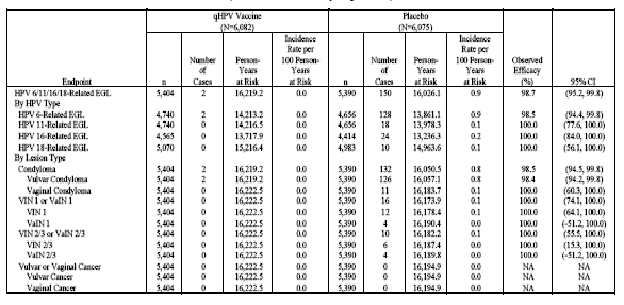
Table 10 . Analysis of Efficacy Against HPV 16/18-Related VIN 2/3 or VaIN 2/3 or Worse
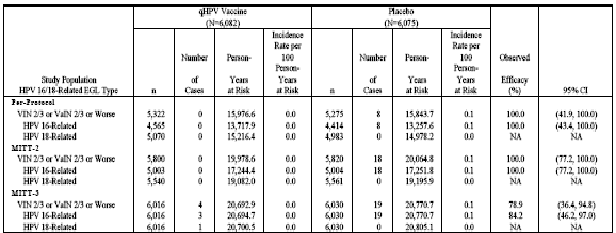
Table 11(Table 11-12 in Reference P015) presents the corresponding analyses of efficacy with respect to HPV 6/11-related EGL.
Table 11 . Analysis of Efficacy Against HPV 6/11-Related External Genital Lesions (Per-Protocol Efficacy Population)
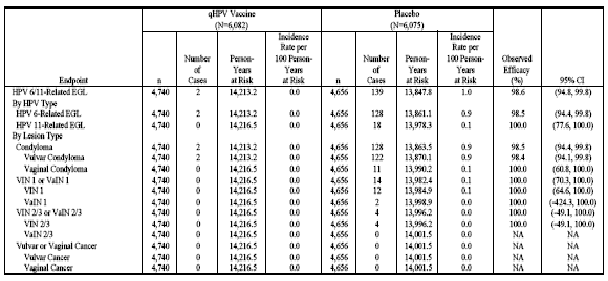
Table 12 (Table 11-15 in Reference P015) shows analyses of population impact with respect to EGLs (caused by vaccine and/or non-vaccine HPV types) in the RMITT-2, MITT-2, and MITT-3 populations.
Table 12 . Analysis of Efficacy Against EGL Due to Any HPV Type by Lesion Type (RMITT-2, MITT-2, and MITT-3)

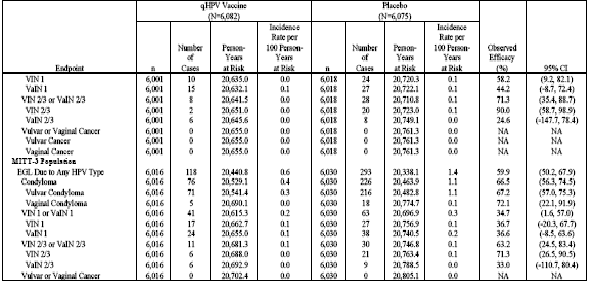
![]()
Results of Combined Studies
The combined incidence of HPV 6-, 11-, 16-, or 18-related genital warts, VIN, or VaIN was evaluated using the combined Protocol 013 and Protocol 015 database (Combined Phase III Database). Results of these analyses for PPE, MITT-2, and MITT-3 are presented in Table 13, Table 14, and Table 15, respectively.
Reviewer’s comments: As mentioned in the previous reviewer’s comment, Protocol 007 data were not included in the combined study analyses. The applicant did not provide tables for combined 013 and 015 data analyses. Table 13 through Table 17 were generated by the reviewer using StatXact (using data presented in References P013 and P015).
Table 13 . Analysis of Efficacy Against HPV 6/11/16/18-Related External Genital Lesions by Severity of Disease (Protocol 013 and 015 Combined, PPE)
|
GARDASIL (N=8799) |
Placebo (N=8800) |
Observed Efficacy (%) |
95% CI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
Study Population HPV 6/11/16/18-Related EGL Type |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
||
Per-Protocol |
|
|
|
|
|
|
|
|
|
|
EGL |
7665 |
2 |
23039.6 |
0.0 |
7669 |
224 |
22802.6 |
1.0 |
99.1 |
(96.8, 99.9) |
Condyloma |
7665 |
2 |
23039.6 |
0.0 |
7669 |
190 |
22849.4 |
0.8 |
99.0 |
(96.2, 99.9) |
Vulvar Condyloma |
7665 |
2 |
23039.6 |
0.0 |
7669 |
182 |
22857.3 |
0.8 |
98.9 |
(96.0, 99.9) |
Vaginal Condyloma |
7665 |
0 |
23042.9 |
0.0 |
7669 |
20 |
23042.8 |
0.1 |
100.0 |
(79.8, 100.0) |
VIN 1 or VaIN 1 |
7665 |
0 |
23042.9 |
0.0 |
7669 |
28 |
23026.7 |
0.1 |
100.0 |
(85.9, 100.0) |
VIN 1 |
7665 |
0 |
23042.9 |
0.0 |
7669 |
16 |
23041.1 |
0.1 |
100.0 |
(74.1, 100.0) |
VaIN 1 |
7665 |
0 |
23042.9 |
0.0 |
7669 |
12 |
23049.8 |
0.1 |
100.0 |
(64.0, 100.0) |
VIN 2/3 or VaIN 2/3 or worse |
7665 |
0 |
23042.9 |
0.0 |
7669 |
23 |
23036.2 |
0.1 |
100.0 |
(82.6, 100.0) |
VIN 2/3 |
7665 |
0 |
23042.9 |
0.0 |
7669 |
13 |
23046.9 |
0.1 |
100.0 |
(67.2, 100.0) |
VaIN 2/3 |
7665 |
0 |
23042.9 |
0.0 |
7669 |
10 |
23053.6 |
0.1 |
100.0 |
(55.4, 100.0) |
Vulvar or Vaginal Cancer |
7665 |
0 |
23042.9 |
0.0 |
7669 |
0 |
23064.1 |
0.0 |
100.0 |
NA |
Vulvar Cancer |
7665 |
0 |
23042.9 |
0.0 |
7669 |
0 |
23064.1 |
0.0 |
100.0 |
NA |
Vaginal Cancer |
7665 |
0 |
23042.9 |
0.0 |
7669 |
0 |
23064.1 |
0.0 |
100.0 |
NA |
Table 14 . Analysis of Efficacy Against HPV 6/11/16/18-Related External Genital Lesions by Severity of Disease (Protocol 013 and 015 Combined, MITT-2)
|
GARDASIL (N=8799) |
Placebo (N=8800) |
Observed Efficacy (%) |
95% CI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
Study Population HPV 6/11/16/18-Related EGL Type |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
||
MITT-2 |
|
|
|
|
|
|
|
|
|
|
EGL |
8496 |
11 |
29109.1 |
0.04 |
8531 |
299 |
28911.8 |
1.03 |
96.3 |
(93.4, 98.2) |
Condyloma |
8496 |
10 |
29109.6 |
0.03 |
8531 |
249 |
28991.9 |
0.86 |
96.0 |
(92.5, 98.1) |
Vulvar Condyloma |
8496 |
8 |
29113.8 |
0.03 |
8531 |
235 |
29013.8 |
0.81 |
96.6 |
(93.2, 98.6) |
Vaginal Condyloma |
8496 |
2 |
29127.2 |
0.01 |
8531 |
28 |
29286.6 |
0.10 |
92.8 |
(71.5, 99.2) |
VIN 1 or VaIN 1 |
8496 |
2 |
29127.2 |
0.01 |
8531 |
38 |
29267.3 |
0.13 |
94.7 |
(79.5, 99.4) |
VIN 1 |
8496 |
2 |
29127.2 |
0.01 |
8531 |
20 |
29294.9 |
0.07 |
89.9 |
(58.6, 98.9) |
VaIN 1 |
8496 |
0 |
29131.4 |
0.00 |
8531 |
18 |
29299.8 |
0.06 |
100.0 |
(77.1, 100.0) |
VIN 2/3 or VaIN 2/3 or worse |
8496 |
1 |
29130.9 |
0.00 |
8531 |
36 |
29274.7 |
0.12 |
97.2 |
(83.4, 99.9) |
VIN 2/3 |
8496 |
1 |
29130.9 |
0.00 |
8531 |
24 |
29288.5 |
0.08 |
95.8 |
(74.3, 99.9) |
VaIN 2/3 |
8496 |
0 |
29131.4 |
0.00 |
8531 |
12 |
29313.4 |
0.04 |
100.0 |
(63.8, 100.0) |
Vulvar or Vaginal Cancer |
8496 |
0 |
29131.4 |
0.00 |
8531 |
0 |
29327.3 |
0.00 |
100.0 |
NA |
Vulvar Cancer |
8496 |
0 |
29131.4 |
0.00 |
8531 |
0 |
29327.3 |
0.00 |
100.0 |
NA |
Vaginal Cancer |
8496 |
0 |
29131.4 |
0.00 |
8531 |
0 |
29327.3 |
0.00 |
100.0 |
NA |
Table 15 . Analysis of Efficacy Against HPV 6/11/16/18-Related External Genital Lesions by Severity of Disease (Protocol 013 and 015 Combined, MITT-3)
|
GARDASIL (N=8799) |
Placebo (N=8800) |
Observed Efficacy (%) |
95% CI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
Study Population HPV 6/11/16/18-Related EGL Type |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
||
MITT-3 |
|
|
|
|
|
|
|
|
|
|
EGL |
8688 |
72 |
29592.7 |
0.24 |
8704 |
362 |
29324.9 |
1.23 |
80.3 |
(74.6, 84.9) |
Condyloma |
8688 |
61 |
29619.7 |
0.21 |
8704 |
304 |
29427.6 |
1.03 |
80.1 |
(73.7, 85.1) |
Vulvar Condyloma |
8688 |
56 |
29633.6 |
0.19 |
8704 |
288 |
29452.3 |
0.98 |
80.7 |
(74.2, 85.8) |
Vaginal Condyloma |
8688 |
5 |
29763.3 |
0.02 |
8704 |
32 |
29812.6 |
0.11 |
84.4 |
(59.5, 95.2) |
VIN 1 or VaIN 1 |
8688 |
12 |
29748.0 |
0.04 |
8704 |
49 |
29811.0 |
0.16 |
75.5 |
(53.2, 88.1) |
VIN 1 |
8688 |
8 |
29759.3 |
0.03 |
8704 |
26 |
29848.9 |
0.09 |
69.1 |
(29.8, 87.9) |
VaIN 1 |
8688 |
4 |
29765.9 |
0.01 |
8704 |
23 |
29858.1 |
0.08 |
82.6 |
(48.9, 95.6) |
VIN 2/3 or VaIN 2/3 or worse |
8688 |
9 |
29758.1 |
0.03 |
8704 |
42 |
29832.5 |
0.14 |
78.5 |
(55.2, 90.8) |
VIN 2/3 |
8688 |
8 |
29760.1 |
0.03 |
8704 |
29 |
29849.4 |
0.10 |
72.3 |
(38.0, 89.1) |
VaIN 2/3 |
8688 |
2 |
29772.3 |
0.01 |
8704 |
14 |
29877.1 |
0.05 |
85.7 |
(37.6, 98.4) |
Vulvar or Vaginal Cancer |
8688 |
0 |
29777.2 |
0.00 |
8704 |
0 |
29896.0 |
0.00 |
100.0 |
NA |
Vulvar Cancer |
8688 |
0 |
29777.2 |
0.00 |
8704 |
0 |
29896.0 |
0.00 |
100.0 |
NA |
Vaginal Cancer |
8688 |
0 |
29777.2 |
0.00 |
8704 |
0 |
29896.0 |
0.00 |
100.0 |
NA |
The impact of GARDASIL on rates of HPV 16-, or 18-related VIN 2/3 and VaIN 2/3 or worse was evaluated in the Combined III Efficacy Database for PPE, MITT-2, and MITT-3 ( Table 16).
Table 16 . Analysis of Efficacy Against HPV 16/18-related VIN 2/3 and VaIN 2/3 (Protocol 013 and 015 Combined)
|
GARDASIL (N=8799) |
Placebo (N=8800) |
Observed Efficacy (%) |
95% CI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
Study Population HPV 6/11/16/18 related VIN 2/3 or VaIN 2/3 or worse |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
||
Per-Protocol |
|
|
|
|
|
|
|
|
|
|
VIN 2/3 or VaIN 2/3 or worse |
7541 |
0 |
22669.0 |
0.00 |
7514 |
19 |
22586.3 |
0.08 |
100.0 |
(78.7, 100.0) |
HPV 16-related |
6455 |
0 |
19417.0 |
0.00 |
6269 |
17 |
18849.6 |
0.09 |
100.0 |
(76.5, 100.0) |
HPV 18-related |
7190 |
0 |
21613.9 |
0.00 |
7119 |
2 |
21419.5 |
0.01 |
100.0 |
(-427.7, 100.0) |
MITT-2 |
|
|
|
|
|
|
|
|
|
|
VIN 2/3 or VaIN 2/3 or worse |
8381 |
1 |
28746.3 |
0.00 |
8415 |
33 |
28890.5 |
0.11 |
97.0 |
(81.8, 99.9) |
HPV 16-related |
7219 |
1 |
24769.9 |
0.00 |
7221 |
31 |
24810.4 |
0.12 |
96.8 |
(80.6, 99.9) |
HPV 18-related |
8021 |
0 |
27520.5 |
0.00 |
8066 |
3 |
27730.0 |
0.01 |
100.0 |
(-143.8, 100.0) |
MITT-3 |
|
|
|
|
|
|
|
|
|
|
VIN 2/3 or VaIN 2/3 or worse |
8688 |
9 |
29758.1 |
0.03 |
8701 |
37 |
29841.8 |
0.12 |
75.6 |
(48.5, 89.7) |
HPV 16-related |
8688 |
8 |
29759.9 |
0.03 |
8701 |
35 |
29845.2 |
0.12 |
77.1 |
(49.7, 90.8) |
HPV 18-related |
8688 |
1 |
29765.7 |
0.00 |
8701 |
3 |
29890.4 |
0.01 |
66.5 |
(-316.9, 99.4) |
The impact of GARDASIL on rates of HPV 6-, or 11-related condyloma was evaluated in the Combined III Efficacy Database for PPE, MITT-2, and MITT-3 ( Table 17).
Table 17 . Analysis of Efficacy Against HPV 6/11-Related Condyloma (Protocols 013 and 015 Combined)
|
GARDASIL (N=8799) |
Placebo (N=8800) |
Observed Efficacy (%) |
95% CI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
Study Population HPV 6/11/16/18-Related Condyloma |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
n |
Number of Cases |
Person-Years at Rick |
Incidence Rate per 100 Person- Years at Rick |
||
Per-Protocol |
|
|
|
|
|
|
|
|
|
|
Condyloma |
6718 |
2 |
20175.7 |
0.01 |
6647 |
186 |
19798.0 |
0.94 |
98.9 |
(96.1, 99.9) |
HPV 6-related |
6718 |
2 |
20175.7 |
0.01 |
6647 |
164 |
19798.0 |
0.83 |
98.8 |
(95.6, 99.9) |
HPV 11-related |
6718 |
0 |
20175.7 |
0.00 |
6647 |
31 |
19798.0 |
0.16 |
100.0 |
(87.6, 100.0) |
MITT-2 |
|
|
|
|
|
|
|
|
|
|
Condyloma |
7528 |
9 |
25795.6 |
0.03 |
7552 |
243 |
25667.3 |
0.95 |
96.3 |
(92.9, 98.3) |
HPV 6-related |
7528 |
8 |
25795.6 |
0.03 |
7552 |
209 |
25667.3 |
0.81 |
96.2 |
(92.4, 98.4) |
HPV 11-related |
7528 |
1 |
25795.6 |
0.00 |
7552 |
46 |
25667.3 |
0.18 |
97.8 |
(87.3, 99.9) |
MITT-3 |
|
|
|
|
|
|
|
|
|
|
Condyloma |
8688 |
60 |
29622.2 |
0.20 |
8701 |
297 |
29440.8 |
1.01 |
79.9 |
(73.4, 85.1) |
HPV 6-related |
8688 |
56 |
29622.2 |
0.19 |
8701 |
260 |
29440.8 |
0.88 |
78.6 |
(71.3, 84.3) |
HPV 11-related |
8688 |
4 |
29622.2 |
0.01 |
8701 |
50 |
29440.8 |
0.17 |
92.0 |
(78.3, 97.9) |
Reviewer’s comments: 1) Results of analyses based on two studies combined (Protocols 013 and 015) are similar to those based on three studies combined (Protocols 007, 013, and 015); 2) Due to low incidence rates of VIN 2/3 and VaIN 2/3 caused by HPV 18, vaccine efficacy for these endpoints were not statistically significant ( a = 0.05).
III. EFFICACY CONCLUSIONS
The efficacy conclusions with respect to vulvar and vaginal HPV disease presented in this application are summarized as follows:
- Prophylactic administration of a 3-dose regimen of GARDASIL to 16- to 26-year old women is effective in preventing the development of VIN 2/3 and VaIN 2/3 caused by HPV 16- and HPV 18.
- Dr. Gerald Willet (from DRUP/CDER) has suggested that labeled indication for prevention of HPV 16 and 18-related vulvar and vaginal cancers is acceptable provided that Gardasil is effective in preventing VIN 2/3 and VaIN 2/3. We acknowledge and abide by his recommendation.
Data presented in this submission confirm the original indication for prevention of VIN 2/3 and VaIN 2/3 caused by HPV 16- and HPV 18. Based on Dr. Gerald Willet’s comments that prevention of VIN 2/3 and VaIN 2/3 can be viewed as acceptable surrogates for prevention of vulvar and vaginal squamous cell cancers, respectively, we recommend prevention of vulvar and vaginal cancers to be included in the label indication with appropriate language, because not all vulvar and vaginal cancers are HPV-related.
V. COMMENTS AND QUESTIONS TO CBER REVIEW COMMITTEE
- Please note that due to low incidence rates of VIN 2/3 and VaIN 2/3 caused by HPV 18, vaccine efficacy for these endpoints were not statistically significant. Efficacy conclusions are based on the composite endpoints.
VI. COMMENTS AND QUESTIONS TO APPLICANT
Given that Protocol 007 did not separate VIN 2/3 and VaIN 2/3 diseases as individual endpoints (they were two components of the composite endpoint) while CBER’s review focused on these specific endpoints, data from Protocol 007 were not included in CBER’s combined study analysis. Please comment.


