Social Security Online
Compilation of the Social Security Laws, Vol II
|
Skip to content Social Security Online |
Compilation of the Social Security Laws, Vol II |
|
|
|
||
* * * * * * *
Sec. 306. [42 U.S.C. 242k]
* * * * * * *
(e) For the purpose of producing comparable and uniform health information and statistics, there is established the Cooperative Health Statistics System. The Secretary, acting through the Center, shall—
(1) coordinate the activities of Federal agencies involved in the design and implementation of the System;
* * * * * * *
Sec. 314.[42 U.S.C. 246]
(a)(1) Authorization.—In order to assist the States in comprehensive and continuing planning for their current and future health needs, the Secretary is authorized during the period beginning July 1, 1966, and ending June 30, 1973, to make grants to States which have submitted, and had approved by the Secretary, State plans for comprehensive State health planning. For the purposes of carrying out this subsection, there are hereby authorized to be appropriated $2,500,000 for the fiscal year ending June 30, 1967, $7,000,000 for the fiscal year ending June 30, 1968, $10,000,000 for the fiscal year ending June 30, 1969, $15,000,000 for the fiscal year ending June 30, 1970, $15,000,000 for the fiscal year ending June 30, 1971, $17,000,000 for the fiscal year ending June 30, 1972, $20,000,000 for the fiscal year ending June 30, 1973, and $10,000,000 for the fiscal year ending June 30, 1974.
(2) State plans for comprehensive state health planning.—In order to be approved for purposes of this subsection, a State plan for comprehensive State health planning must—
(A) designate, or provide for the establishment of, a single State agency, which may be an interdepartmental agency, as the sole agency for administering or supervising the administration of the State's health planning functions under the plan;
(B) provide for the establishment of a State health planning council, which shall include representatives of Federal, State, and local agencies (including as an ex officio member, if there is located in such State one or more hospitals or other health care facilities of the Department of Veterans Affairs the individual whom the Secretary of Veterans Affairs shall have designated to serve on such council as the representative of the hospitals or other health care facilities of such Department which are located in such State) and nongovernmental organizations and groups concerned with health (including representation of the regional medical program or programs included in whole or in part within the State), and of consumers of health services, to advise such State agency in carrying out its functions under the plan, and a majority of the membership of such council shall consist of representatives of consumers of health services;
(C) set forth policies and procedures for the expenditure of funds under the plan, which, in the judgment of the Secretary, are designed to provide for comprehensive State planning for health services (both public and private and including home health care), including the facilities and persons required for the provision of such services, to meet the health needs of the people of the State and including environmental considerations as they relate to public health;
(D) provide for encouraging cooperative efforts among governmental or nongovernmental agencies, organizations and groups concerned with health services, facilities, or manpower, and for cooperative efforts between such agencies, organizations, and groups and similar agencies, organizations, and groups in the fields of education, welfare, and rehabilitation;
(E) contain or be supported by assurances satisfactory to the Secretary that the funds paid under this subsection will be used to supplement and, to the extent practicable, to increase the level of funds that would otherwise be made available by the State for the purpose of comprehensive health planning and not to supplant such non-Federal funds;
(F)[1] provide such methods of administration (including methods relating to the establishment and maintenance of personnel standards on a merit basis, except that the Secretary shall exercise no authority with respect to the selection, tenure of office, and compensation of any individual employed in accordance with such methods) as are found by the Secretary to be necessary for the proper and efficient operation of the plan;
(G) provide that the State agency will make such reports, in such form and containing such information, as the Secretary may from time to time reasonably require, and will keep such records and afford such access thereto as the Secretary finds necessary to assure the correctness and verification of such reports;
(H) provide that the State agency will from time to time, but not less often than annually, review its State plan approved under this subsection and submit to the Secretary appropriate modifications thereof;
(I) effective July 1, 1968, (i) provide for assisting each health care facility in the State to develop a program for capital expenditures for replacement, modernization, and expansion which is consistent with an overall State plan developed in accordance with criteria established by the Secretary after consultation with the State which will meet the needs of the State for health care facilities, equipment, and services without duplication and otherwise in the most efficient and economical manner, and (ii) provide that the State agency furnishing such assistance will periodically review the program (developed pursuant to clause (i)) of each health care facility in the State and recommend appropriate modification thereof;
(J) provide for such fiscal control and fund accounting procedures as may be necessary to assure proper disbursement of and accounting for funds paid to the State under this subsection; and
(K) contain such additional information and assurances as the Secretary may find necessary to carry out the purposes of this subsection.
(3)(A) State allotments.—From the sums appropriated for such purpose for each fiscal year, the several States shall be entitled to allotments determined, in accordance with regulations, on the basis of the population and the per capita income of the respective States; except that no such allotment to any State for any fiscal year shall be less than 1 per centum of the sum appropriated for such fiscal year pursuant to paragraph (1). Any such allotment to a State for a fiscal year shall remain available for obligation by the State, in accordance with the provisions of this subsection and the State's plan approved thereunder, until the close of the succeeding fiscal year.
(B) The amount of any allotment to a State under subparagraph (A) for any fiscal year which the Secretary determines will not be required by the State, during the period for which it is available, for the purposes for which allotted shall be available for reallotment by the Secretary from time to time, on such date or dates as he may fix, to other States with respect to which such a determination has not been made, in proportion to the original allotments to such States under subparagraph (A) for such fiscal year, but with such proportionate amount for any of such other States being reduced to the extent it exceeds the sum the Secretary estimates such State needs and will be able to use during such period; and the total of such reductions shall be similarly reallotted among the States whose proportionate amounts were not so reduced. Any amount so reallotted to a State from funds appropriated pursuant to this subsection for a fiscal year shall be deemed part of its allotment under subparagraph (A) for such fiscal year.
(4) Payments to states.—From each State's allotment for a fiscal year under this subsection, the State shall from time to time be paid the Federal share of the expenditures incurred during that year or the succeeding year pursuant to its State plan approved under this subsection. Such payments shall be made on the basis of estimates by the Secretary of the sums the State will need in order to perform the planning under its approved State plan under this subsection, but with such adjustments as may be necessary to take account of previously made underpayments or overpayments. The “Federal share” for any State for purposes of this subsection shall be all, or such part as the Secretary may determine, of the cost of such planning, except that in the case of the allotments for the fiscal year ending June 30, 1970, it shall not exceed 75 per centum of such cost.
* * * * * * *
Sec. 317.[42 U.S.C. 247b]
(j) Authorization of Appropriations
* * * * * * *
(1) Except for grants for immunization programs the authorization of appropriations for which are established in paragraph (2), for grants under subsections (a) and (k)(1) of this section for preventive health service programs to immunize without charge children, adolescents, and adults against vaccine-preventable diseases, there are authorized such sums as may be necessary for each of the fiscal years 1998 through 2005. Not more than 10 percent of the total amount appropriated under the preceding sentence for any fiscal year shall be available for grants under subsection (k)(1) of this section for such fiscal year.
(B) For grants under subsection (a) of this section for preventive health service programs for the provision without charge of immunizations with vaccines approved for use, and recommended for routine use, after October 1, 1997, there are authorized to be appropriated such sums as may be necessary.
* * * * * * *
Sec. 317 A. [42 U.S.C. 247b-1]
(a) Authority for Grants.—
(1) In general.—Subject to paragraph (2), the Secretary, acting through the Director of the Centers for Disease Control and Prevention, may make grants to States and political subdivisions of States for the initiation and expansion of community programs designed—
(A) to provide, for infants and children—
(i) screening for elevated blood lead levels;
(ii) referral for treatment of such levels; and
(iii) referral for environmental intervention associated with such levels; and
(B) to provide education about childhood lead poisoning.
(2) Authority regarding certain entities.—With respect to a geographic area with a need for activities authorized in paragraph (1), in any case in which neither the State nor the political subdivision in which such area is located has applied for a grant under paragraph (1), the Secretary may make a grant under such paragraph to any grantee under section 329, 330, 340, or 340A for carrying out such activities in the area.
(3) Provision of all services and activities through each grantee.—In making grants under paragraph (1), the Secretary shall ensure that each of the activities described in such paragraph is provided through each grantee under such paragraph. The Secretary may authorize such a grantee to provide the services and activities directly, or through arrangements with other providers.
(b) Status as Medicaid Provider.—
(1) In general.—Subject to paragraph (2), the Secretary may not make a grant under subsection (a) unless, in the case of any service described in such subsection that is made available pursuant to the State plan approved under title XIX of the Social Security Act for the State involved—
(A) the applicant for the grant will provide the service directly, and the applicant has entered into a participation agreement under the State plan and is qualified to receive payments under such plan; or
(B) the applicant will enter into an agreement with a provider under which the provider will provide the service, and the provider has entered into such a participation agreement and is qualified to receive such payments.
(2) Waiver regarding certain secondary agreements.—
(A) In the case of a provider making an agreement pursuant to paragraph (1)(B) regarding the provision of services, the requirement established in such paragraph regarding a participation agreement shall be waived by the Secretary if the provider does not, in providing health care services, impose a charge or accept reimbursement available from any third-party payor, including reimbursement under any insurance policy or under any Federal or State health benefits plan.
(B) A determination by the Secretary of whether a provider referred to in subparagraph (A) meets the criteria for a waiver under such subparagraph shall be made without regard to whether the provider accepts voluntary donations regarding the provision of services to the public.
(c) Priority in Making Grants.—In making grants under subsection (a), the Secretary shall give priority to applications for programs that will serve areas with a high incidence of elevated blood lead levels in infants and children.
(d) Grant Application.—No grant may be made under subsection (a), unless an application therefor has been submitted to, and approved by, the Secretary. Such an application shall be in such form and shall be submitted in such manner as the Secretary shall prescribe and shall include each of the following:
(1) A complete description of the program which is to be provided by or through the applicant.
(2) Assurances satisfactory to the Secretary that the program to be provided under the grant applied for will include educational programs designed to—
(A) communicate to parents, educators, and local health officials the significance and prevalence of lead poisoning in infants and children (including the sources of lead exposure, the importance of screening young children for lead, and the preventive steps that parents can take in reducing the risk of lead poisoning) which the program is designed to detect and prevent; and
(B) communicate to health professionals and paraprofessionals updated knowledge concerning lead poisoning and research (including the health consequences, if any, of low-level lead burden; the prevalence of lead poisoning among all socioeconomic groupings; the benefits of expanded lead screening; and the therapeutic and other interventions available to prevent and combat lead poisoning in affected children and families).
(3) Assurances satisfactory to the Secretary that the applicant will report on a quarterly basis the number of infants and children screened for elevated blood lead levels, the number of infants and children who were found to have elevated blood lead levels, the number and type of medical referrals made for such infants and children, the outcome of such referrals, and other information to measure program effectiveness.
(4) Assurances satisfactory to the Secretary that the applicant will make such reports respecting the program involved as the Secretary may require.
(5) Assurances satisfactory to the Secretary that the applicant will coordinate the activities carried out pursuant to subsection (a) with related activities and services carried out in the State by grantees under title V or XIX of the Social Security Act.
(6) Assurances satisfactory to the Secretary that Federal funds made available under such a grant for any period will be so used as to supplement and, to the extent practical, increase the level of State, local, and other non-Federal funds that would, in the absence of such Federal funds, be made available for the program for which the grant is to be made and will in no event supplant such State, local, and other non-Federal funds.
(7) Assurances satisfactory to the Secretary that the applicant will ensure complete and consistent reporting of all blood lead test results from laboratories and health care providers to State and local health departments in accordance with guidelines of the Centers for Disease Control and Prevention for standardized reporting as described in subsection (m) of this section.
(8) Such other information as the Secretary may prescribe.
* * * * * * *
Sec. 332. [42 U.S.C. 254e]
(a)(1) For purposes of this subpart the term “health professional shortage area” means (A) an area in an urban or rural area (which need not conform to the geographic boundaries of a political subdivision and which is a rational area for the delivery of health services) which the Secretary determines has a health manpower shortage and which is not reasonably accessible to an adequately served area, (B) a population group which the Secretary determines has such a shortage, or (C) a public or nonprofit private medical facility or other public facility which the Secretary determines has such a shortage. All Federally qualified health centers and rural health clinics, as defined in section 1861(aa) of the Social Security Act (42 U.S.C. 1395x(aa)), that meet the requirements of section 254g of this title shall be automatically designated as having such a shortage. Not earlier than 6 years after such date of designation, and every 6 years thereafter, each such center or clinic shall demonstrate that the center or clinic meets the applicable requirements of the Federal regulations, regarding the definition of a health professional shortage area for purposes of this section. The Secretary shall not remove an area from the areas determined to be health professional shortage areas under subparagraph (A) of the preceding sentence until the Secretary has afforded interested persons and groups in such area an opportunity to provide data and information in support of the designation as a health professional shortage area or a population group described in subparagraph (B) of such sentence or a facility described in subparagraph (C) of such sentence, and has made a determination on the basis of the data and information submitted by such persons and groups and other data and information available to the Secretary.
Sec. 338E. [42 U.S.C. 254o]
(a)(1) An individual who has entered into a written contract with the Secretary under section 338A and who—
(A) fails to maintain an acceptable level of academic standing in the educational institution in which he is enrolled (such level determined by the educational institution under regulations of the Secretary); or
(B) is dismissed from such educational institution for disciplinary reasons; or
(C) voluntarily terminates the training in such an educational institution for which he is provided a scholarship under such contract, before the completion of such training,
in lieu of any service obligation arising under such contract, shall be liable to the United States for the amount which has been paid to him, or on his behalf, under the contract.
(2) An individual who has entered into a written contract with the Secretary under section 338B and who—
(A) in the case of an individual who is enrolled in the final year of a course of study, fails to maintain an acceptable level of academic standing in the educational institution in which such individual is enrolled (such level determined by the educational institution under regulations of the Secretary) or voluntarily terminates such enrollment or is dismissed from such educational institution before completion of such course of study; or
(B) in the case of an individual who is enrolled in a graduate training program, fails to complete such training program and does not receive a waiver from the Secretary under section 338B(b)(1)(B)(ii),
in lieu of any service obligation arising under such contract shall be liable to the United States for the amount that has been paid on behalf of the individual under the contract.
(b)(1)(A) Except as provided in paragraph (2), if an individual breaches his written contract by failing (for any reason not specified in subsection (a) or section 338F(d)) to begin such individual’s service obligation under section 338A in accordance with section 338C or 338D to complete such service obligation under section 338A, or to complete a required residency as specified in section 338B, the United States shall be entitled to recover from the individual an amount determined in accordance with the formula
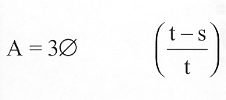
in which “A” is the amount the United States is entitled to recover, “” is the sum of the amounts paid under this subpart to or on behalf of the individual and the interest on such amounts which would be payable if at the time the amounts were paid they were loans bearing interest at the maximum legal prevailing rate, as determined by the Treasurer of the United States; “t” is the total number of months in the individual’s period of obligated service; and “s” is the number of months of such period served by him in accordance with section 338C or a written agreement under section 338D.
(B)(i) Any amount of damages that the United States is entitled to recover under this subsection or under subsection (c) shall, within the 1-year period beginning on the date of the breach of the written contract (or such longer period beginning on such date as specified by the Secretary), be paid to the United States. Amounts not paid within such period shall be subject to collection through deductions in Medicare payments pursuant to section 1892 of the Social Security Act.
(ii) If damages described in clause (i) are delinquent for 3 months, the Secretary shall, for the purpose of recovering such damages—
(I) utilize collection agencies contracted with by the Administrator of the General Services Administration; or
(II) enter into contracts for the recovery of such damages with collection agencies selected by the Secretary.
(iii) Each contract for recovering damages pursuant to this subsection shall provide that the contractor will, not less than once each 6 months, submit to the Secretary a status report on the success of the contractor in collecting such damages. Section 3718 of title 31, United States Code, shall apply to any such contract to the extent not inconsistent with this subsection.
(iv) To the extent not otherwise prohibited by law, the Secretary shall disclose to all appropriate credit reporting agencies information relating to damages of more than $100 that are entitled to be recovered by the United States under this subsection and that are delinquent by more than 60 days or such longer period as is determined by the Secretary.
(2) If an individual is released under section 753 from a service obligation under section 225 (as in effect on September 30, 1977) and if the individual does not meet the service obligation incurred under section 753, subsection (f) of such section 225 shall apply to such individual in lieu of paragraph (1) of this subsection.
(3) The Secretary may terminate a contract with an individual under section 338A if, not later than 30 days before the end of the school year to which the contract pertains, the individual—
(A) submits a written request for such termination; and
(B) repays all amounts paid to, or on behalf of, the individual under section 338A(g).
(c)(1) If (for any reason not specified in subsection (a) or section 338G(d)) an individual breaches the written contract of the individual under section 338B by failing either to begin such individual's service obligation in accordance with section 338C or 338D or to complete such service obligation, the United States shall be entitled to recover from the individual an amount equal to the sum of—
(A) the total of the amounts paid by the United States under section 338B(g) on behalf of the individual for any period of obligated service not served;
(B) an amount equal to the product of the number of months of obligated service that were not completed by the individual, multiplied by $7,500; and
(C) the interest on the amounts described in subparagraphs (A) and (B), at the maximum legal prevailing rate, as determined by the Treasurer of the United States, from the date of the breach;
except that the amount the United States is entitled to recover under this paragraph shall not be less than $31,000.
(2) The Secretary may terminate a contract with an individual under section 338B if, not later than 45 days before the end of the fiscal year in which the contract was entered into, the individual—
(A) submits a written request for such termination; and
(B) repays all amounts paid on behalf of the individual under section 338B(g).
(3) Damages that the United States is entitled to recover shall be paid in accordance with subsection (b)(1)(B).
(d)(1) Any obligation of an individual under the Scholarship Program (or a contract thereunder) or the Loan Repayment Program (or a contract thereunder) for service or payment of damages shall be canceled upon the death of the individual.
(2) The Secretary shall by regulation provide for the partial or total waiver or suspension of any obligation of service or payment by an individual under the Scholarship Program (or a contract thereunder) or the Loan Repayment Program (or a contract thereunder) whenever compliance by the individual is impossible or would involve extreme hardship to the individual and if enforcement of such obligation with respect to any individual would be unconscionable.
(3)(A) Any obligation of an individual under the Scholarship Program (or a contract thereunder) or the Loan Repayment Program (or a contract thereunder) for payment of damages may be released by a discharge in bankruptcy under title 11 of the United States Code only if such discharge is granted after the expiration of the 7-year period beginning on the first date that payment of such damages is required, and only if the bankruptcy court finds that nondischarge of the obligation would be unconscionable.
(B)(i) Subparagraph (A) shall apply to any financial obligation of an individual under the provision of law specified in clause (ii) to the same extent and in the same manner as such subparagraph applies to any obligation of an individual under the Scholarship or Loan Repayment Program (or contract thereunder) for payment of damages.
(ii) The provision of law referred to in clause (i) is subsection (f) of section 225 of this Act, as in effect prior to the repeal of such section by section 408(b)(1) of Public Law 94-484.
(e) Notwithstanding any other provision of Federal or State law, there shall be no limitation on the period within which suit may be filed, a judgment may be enforced, or an action relating to an offset or garnishment, or other action, may be initiated or taken by the Secretary, the Attorney General, or the head of another Federal agency, as the case may be, for the repayment of the amount due from an individual under this section.
* * * * * * *
Sec. 339.[42 U.S.C. 255]
(a)(1) For the purpose of encouraging the establishment and initial operation of home health programs to provide home health services in areas in which such services are inadequate or not readily accessible, the Secretary may, in accordance with the provisions of this section, make grants to public and nonprofit private entities and loans to proprietary entities to meet the initial costs of establishing and operating such home health programs. Such grants and loans may include funds to provide training for paraprofessionals (including homemaker home health aides) to provide home health services.
(2) In making grants and loans under this subsection, the Secretary shall—
(A) consider the relative needs of the several States for home health services;
(B) give preference to areas in which a high percentage of the population proposed to be served is composed of individuals who are elderly, medically indigent, or disabled; and
(C) give special consideration to areas with inadequate means of transportation to obtain necessary health services.
(3)(A) No loan may be made to a proprietary entity under this section unless the application of such entity for such loan contains assurances satisfactory to the Secretary that—
(i) at the time the application is made the entity is fiscally sound;
(ii) the entity is unable to secure a loan for the project for which the application is submitted from non-Federal lenders at the rate of interest prevailing in the area in which the entity is located; and
(iii) during the period of the loan, such entity will remain fiscally sound.
(B) Loans under this section shall be made at an interest rate comparable to the rate of interest prevailing on the date the loan is made with respect to the marketable obligations of the United States of comparable maturities, adjusted to provide for administrative costs.
(4) Applications for grants and loans under this subsection shall be in such form and contain such information as the Secretary shall prescribe.
(5) There are authorized to be appropriated for grants and loans under this subsection $5,000,000 for each of the fiscal years ending on September 30, 1983, September 30, 1984, September 30, 1985, September 30, 1986, and September 30, 1987.
(b)(1) The Secretary may make grants to and enter into contracts with public and private entities to assist them in developing appropriate training programs for paraprofessionals (including homemaker home health aides) to provide home health services.
(2) Any program established with a grant or contract under this subsection to train homemaker home health aides shall—
(A) extend for at least forty hours, and consist of classroom instruction and at least twenty hours (in the aggregate) of supervised clinical instruction directed toward preparing students to deliver home health services;
(B) be carried out under appropriate professional supervision and be designed to train students to maintain or enhance the personal care of an individual in his home in a manner which promotes the functional independence of the individual; and
(C) include training in—
(i) personal care services designed to assist an individual in the activities of daily living such as bathing, exercising, personal grooming, and getting in and out of bed; and
(ii) household care services such as maintaining a safe living environment, light housekeeping, and assisting in providing good nutrition (by the purchasing and preparation of food).
(3) In making grants and entering into contracts under this subsection, special consideration shall be given to entities which establish or will establish programs to provide training for persons fifty years of age and older who wish to become paraprofessionals (including homemaker home health aides) to provide home health services.
(4) Applications for grants and contracts under this subsection shall be in such form and contain such information as the Secretary shall prescribe.
(5) There are authorized to be appropriated for grants and contracts under this subsection $2,000,000 for each of the fiscal years ending September 30, 1983, September 30, 1984, September 30, 1985, September 30, 1986, and September 30, 1987.
(c) The Secretary shall report to the Committee on Labor and Human Resources of the Senate and the Committee on Energy and Commerce of the House of Representatives on or before January 1, 1984, with respect to—
(1) the impact of grants made and contracts entered into under subsections (a) and (b) (as such subsections were in effect prior to October 1, 1981);
(2) the need to continue grants and loans under subsections (a) and (b) (as such subsections are in effect on the day after the date of enactment of the Orphan Drug Act[2]); and
(3) the extent to which standards have been applied to the training of personnel who provide home health services.
(d) For purposes of this section, the term “home health services” has the meaning prescribed for the term by section 1861(m) of the Social Security Act.
Sec. 340B. [42 U. S. C. 256b]
(a) Requirements for Agreement With Secretary.—
(1) In General.—The Secretary shall enter into an agreement with each manufacturer of covered drugs under which the amount required to be paid (taking into account any rebate or discount, as provided by the Secretary) to the manufacturer for covered drugs (other than drugs described in paragraph (3)) purchased by a covered entity on or after the first day of the first month that begins after the date of the enactment of this section, does not exceed an amount equal to the average manufacturer price for the drug under title XIX of the Social Security Act in the preceding calendar quarter, reduced by the rebate percentage described in paragraph (2).
(2) Rebate percentage defined.—
(A) In general.—For a covered outpatient drug purchased in a calendar quarter, the “rebate percentage” is the amount (expressed as a percentage) equal to—
(i) the average total rebate required under section 1927(c) of the Social Security Act with respect to the drug (for a unit of the dosage form and strength involved) during the preceding calendar quarter; divided by
(ii) the average manufacturer price for such a unit of the drug during such quarter.
(B) Over the counter drugs.—
(i) In general.—For purposes of subparagraph (A), in the case of over the counter drugs, the “rebate percentage” shall be determined as if the rebate required under section 1927(c) of the Social Security Act is based on the applicable percentage provided under section 1927(c)(4) of such Act.
(ii) Definition.—The term “over the counter drug” means a drug that may be sold without a prescription and which is prescribed by a physician (or other persons authorized to prescribe such drug under State law).
(3) Drugs provided under state medicaid plans.—Drugs described in this paragraph are drugs purchased by the entity for which payment is made by the State under the State plan for medical assistance under title XIX of the Social Security Act.
(4) Covered entity defined.—In this section, the term “covered entity” means an entity that meets the requirements described in paragraph (5) and is one of the following:
(A) A Federally-qualified health center (as defined in section 1905(l)(2)(B) of the Social Security Act).
(B) An entity receiving a grant under section 340A.
(C) A family planning project receiving a grant or contract under section 1001.
(D) An entity receiving a grant under subpart II of part C of title XXVI (relating to categorical grants for outpatient early intervention services for HIV disease).
(E) A State-operated AIDS drug purchasing assistance program receiving financial assistance under title XXVI.
(F) A black lung clinic receiving funds under section 427(a) of the Black Lung Benefits Act.
(G) A comprehensive hemophilia diagnostic treatment center receiving a grant under section 501(a)(2) of the Social Security Act.
(H) A Native Hawaiian Health Center receiving funds under the Native Hawaiian Health Care Act of 1988.
(I) An urban Indian organization receiving funds under title V of the Indian Health Care Improvement Act.
(J) Any entity receiving assistance under title XXVI (other than a State or unit of local government or an entity described in subparagraph (D)), but only if the entity is certified by the Secretary pursuant to paragraph (7).
(K) An entity receiving funds under section 318 (relating to treatment of sexually transmitted diseases) or section 317(j)(2) (relating to treatment of tuberculosis) through a State or unit of local government, but only if the entity is certified by the Secretary pursuant to paragraph (7).
(L) A subsection (d) hospital (as defined in section 1886(d)(1)(B) of the Social Security Act) that—
(i) is owned or operated by a unit of State or local government, is a public or private non-profit corporation which is formally granted governmental powers by a unit of State or local government, or is a private non-profit hospital which has a contract with a State or local government to provide health care services to low income individuals who are not entitled to benefits under title XVIII of the Social Security Act or eligible for assistance under the State plan under this title;
(ii) for the most recent cost of reporting period that ended before the calendar quarter involved, had a disproportionate share adjustment percentage (as determined under section 1886(d)(5)(F) of the Social Security Act) greater than 11.75 percent or was described in section 1886(d)(5)(F)(i)(II) of such Act; and
(iii) does not obtain covered outpatient drugs through a group purchasing organization or other group purchasing arrangement.
(5) Requirements for covered entities.—
(A) Prohibiting duplicate discounts or rebates.—
(i) In general.—A covered entity shall not request payment under title XIX of the Social Security Act for medical assistance described in section 1905(a)(12) of such Act with respect to a drug that is subject to an agreement under this section if the drug is subject to the payment of a rebate to the State under section 1927 of such Act.
(ii) Establishment of mechanism.—The Secretary shall establish a mechanism to ensure that covered entities comply with clause (i). If the Secretary does not establish a mechanism within 12 months under the previous sentence, the requirements of section 1927(a)(5)(C) of the Social Security Act shall apply.
(B) Prohibiting resale of drugs.—With respect to any covered outpatient drug that is subject to an agreement under this subsection, a covered entity shall not resell or otherwise transfer the drug to a person who is not a patient of the entity.
(C) Auditing.—A covered entity shall permit the Secretary and the manufacturer of a covered outpatient drug that is subject to an agreement under this subsection with the entity (acting in accordance with procedures established by the Secretary relating to the number, duration, and scope of audits) to audit at the Secretary's or the manufacturer's expense the records of the entity that directly pertain to the entity's compliance with the requirements described in subparagraphs (A) or (B) with respect to drugs of the manufacturer.
(D) Additional sanction for noncompliance.—If the Secretary finds, after notice and hearing, that a covered entity is in violation of a requirement described in subparagraphs (A) or (B), the covered entity shall be liable to the manufacturer of the covered outpatient drug that is the subject of the violation in an amount equal to the reduction in the price of the drug (as described in subparagraph (A)) provided under the agreement between the entity and the manufacturer under this paragraph.
(6) Treatment of distinct units of hospitals.—In the case of a covered entity that is a distinct part of a hospital, the hospital shall not be considered a covered entity under this paragraph unless the hospital is otherwise a covered entity under this subsection.
(7) Certification of certain covered entities.—
(A) Development of process.—Not later than 60 days after the date of enactment of this subsection, the Secretary shall develop and implement a process for the certification of entities described in subparagraphs (J) and (K) of paragraph (4).
(B) Inclusion of purchase information.—The process developed under subparagraph (A) shall include a requirement that an entity applying for certification under this paragraph submit information to the Secretary concerning the amount such entity expended for covered outpatient drugs in the preceding year so as to assist the Secretary in evaluating the validity of the entity's subsequent purchases of covered outpatient drugs at discounted prices.
(C) Criteria.—The Secretary shall make available to all manufacturers of covered outpatient drugs a description of the criteria for certification under this paragraph.
(D) List of purchasers and dispensers.—The certification process developed by the Secretary under subparagraph (A) shall include procedures under which each State shall, not later than 30 days after the submission of the descriptions under subparagraph (C), prepare and submit a report to the Secretary that contains a list of entities described in subparagraphs (J) and (K) of paragraph (4) that are located in the State.
(E) Recertification.—The Secretary shall require the recertification of entities certified pursuant to this paragraph on a not more frequent than annual basis, and shall require that such entities submit information to the Secretary to permit the Secretary to evaluate the validity of subsequent purchases by such entities in the same manner as that required under subparagraph (B).
(8) Development of prime vendor program.—The Secretary shall establish a prime vendor program under which covered entities may enter into contracts with prime vendors for the distribution of covered outpatient drugs. If a covered entity obtains drugs directly from a manufacturer, the manufacturer shall be responsible for the costs of distribution.
(9) Notice to manufacturers.—The Secretary shall notify manufacturers of covered outpatient drugs and single State agencies under section 1902(a)(5) of the Social Security Act of the identities of covered entities under this paragraph, and of entities that no longer meet the requirements of paragraph (5) or that are no longer certified pursuant to paragraph (7).
(10) No prohibition on larger discount.—Nothing in this subsection shall prohibit a manufacturer from charging a price for a drug that is lower than the maximum price that may be charged under paragraph (1).
(b) Other Definitions.—In this section, the terms “average manufacturer price”, “covered outpatient drug”, and “manufacturer” have the meaning given such terms in section 1927(k) of the Social Security Act.
(c) References to Social Security Act.—Any reference in this section to a provision of the Social Security Act shall be deemed to be a reference to the provision as in effect on the date of the enactment of this section[3].
(d) Compliance With Requirements.—A manufacturer is deemed to meet the requirements of subsection (a) if the manufacturer establishes to the satisfaction of the Secretary that the manufacturer would comply (and has offered to comply) with the provisions of this section (as in effect immediately after the enactment of the Veterans Health Care Act of 1992), as applied by the Secretary, and would have entered into an agreement under this section (as such section was in effect at such time), but for a legislative change in this section (or the application of this section) after the date of the enactment of such Act.
* * * * * * *
Sec. 353.[42 U.S.C. 263a]
(a) Definition.—As used in this section, the term “laboratory” or “clinical laboratory” means a facility for the biological, microbiological, serological, chemical, immuno-hematological, hematological, biophysical, cytological, pathological, or other examination of materials derived from the human body for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or impairment of, or the assessment of the health of, human beings.
(b) Certificate Requirement.—No person may solicit or accept materials derived from the human body for laboratory examination or other procedure unless there is in effect for the laboratory a certificate issued by the Secretary under this section applicable to the category of examinations or procedures which includes such examination or procedure.
(c) Issuance and Renewal of Certificates.—
(1) In general.—The Secretary may issue or renew a certificate for a laboratory only if the laboratory meets the requirements of subsection (d).
(2) Term.—A certificate issued under this section shall be valid for a period of 2 years or such shorter period as the Secretary may establish.
(d) Requirements for Certificates.—
(1) In general.—A laboratory may be issued a certificate or have its certificate renewed if—
(A) the laboratory submits (or if the laboratory is accredited under subsection (e), the accreditation body which accredited the laboratory submits), an application—
(i) in such form and manner as the Secretary shall prescribe,
(ii) that describes the characteristics of the laboratory examinations and other procedures performed by the laboratory including—
(I) the number and types of laboratory examinations and other procedures performed,
(II) the methodologies for laboratory examinations and other procedures employed, and
(III) the qualifications (educational background, training, and experience) of the personnel directing and supervising the laboratory and performing the laboratory examinations and other procedures, and
(iii) that contains such other information as the Secretary may require to determine compliance with this section, and
the laboratory agrees to provide to the Secretary (or if the laboratory is accredited, to the accreditation body which accredited it) a description of any change in the information submitted under clause (ii) not later than 6 months after the change was put into effect,
(B) the laboratory provides the Secretary—
(i) with satisfactory assurances that the laboratory will be operated in accordance with standards issued by the Secretary under subsection (f), or
(ii) with proof of accreditation under subsection (e),
(C) the laboratory agrees to permit inspections by the Secretary under subsection (g),
(D) the laboratory agrees to make records available and submit reports to the Secretary as the Secretary may reasonably require, and
(E) the laboratory agrees to treat proficiency testing samples in the same manner as it treats materials derived from the human body referred to it for laboratory examinations or other procedures in the ordinary course of business.
(2) Requirements for certificates of waiver.—
(A) In general.—A laboratory which only performs laboratory examinations and procedures described in paragraph (3) shall be issued a certificate of waiver or have its certificate of waiver renewed if—
(i) the laboratory submits an application—
(I) in such form and manner as the Secretary shall prescribe,
(II) that describes the characteristics of the laboratory examinations and other procedures performed by the laboratory, including the number and types of laboratory examinations and other procedures performed, the methodologies for laboratory examinations and other procedures employed, and the qualifications (educational background, training, and experience) of the personnel directing and supervising the laboratory and performing the laboratory examinations and other procedures, and
(III) that contains such other information as the Secretary may reasonably require to determine compliance with this section, and
(ii) the laboratory agrees to make records available and submit reports to the Secretary as the Secretary may require.
(B) Changes.—If a laboratory makes changes in the examinations and other procedures performed by it only with respect to examinations and procedures which are described in paragraph (3), the laboratory shall report such changes to the Secretary not later than 6 months after the change has been put into effect. If a laboratory proposes to make changes in the examinations and procedures performed by it such that the laboratory will perform an examination or procedure not described in paragraph (3), the laboratory shall report such change to the Secretary before the change takes effect.
(C) Effect.—Subsections (f) and (g) shall not apply to a laboratory to which has been issued a certificate of waiver.
(3) Examinations and procedures.—The examinations and procedures identified in paragraph (2) are laboratory examinations and procedures that have been approved by the Food and Drug Administration for home use or that, as determined by the Secretary, are simple laboratory examinations and procedures that have an insignificant risk of an erroneous result, including those that
(A) employ methodologies that are so simple and accurate as to render the likelihood of erroneous results by the user negligible, or
(C) the Secretary has determined pose no unreasonable risk of harm to the patient if performed incorrectly.
(4) Definition.—As used in this section, the term “certificate” includes a certificate of waiver issued under paragraph (2).
(e) Accreditation.—
(1) In general.—A laboratory may be accredited for purposes of obtaining a certificate if the laboratory—
(A) meets the standards of an approved accreditation body, and
(B) authorizes the accreditation body to submit to the Secretary (or such State agency as the Secretary may designate) such records or other information as the Secretary may require.
(2) Approval of accreditation bodies.—
(A) In general.—The Secretary may approve a private nonprofit organization to be an accreditation body for the accreditation of laboratories if—
(i) using inspectors qualified to evaluate the methodologies used by the laboratories in performing laboratory examinations and other procedures, the accreditation body agrees to inspect a laboratory for purposes of accreditation with such frequency as determined by Secretary,
(ii) the standards applied by the body in determining whether or not to accredit a laboratory are equal to or more stringent than the standards issued by the Secretary under subsection (f),
(iii) there is adequate provision for assuring that the standards of the accreditation body continue to be met by the laboratory,
(iv) in the case of any laboratory accredited by the body which has had its accreditation denied, suspended, withdrawn, or revoked or which has had any other action taken against it by the accrediting body, the accrediting body agrees to submit to the Secretary the name of such laboratory within 30 days of the action taken,
(v) the accreditation body agrees to notify the Secretary at least 30 days before it changes its standards, and
(vi) if the accreditation body has its approval withdrawn by the Secretary, the body agrees to notify each laboratory accredited by the body of the withdrawal within 10 days of the withdrawal.
(B) Criteria and procedures.—The Secretary shall promulgate criteria and procedures for approving an accreditation body and for withdrawing such approval if the Secretary determines that the accreditation body does not meet the requirements of subparagraph (A).
(C) Effect of withdrawal of approval.—If the Secretary withdraws the approval of an accreditation body under subparagraph (B), the certificate of any laboratory accredited by the body shall continue in effect for 60 days after the laboratory receives notification of the withdrawal of the approval, except that the Secretary may extend such period for a laboratory if it determines that the laboratory submitted an application for accreditation or a certificate in a timely manner after receipt of the notification of the withdrawal of approval. If an accreditation body withdraws or revokes the accreditation of a laboratory, the certificate of the laboratory shall continue in effect—
(i) for 45 days after the laboratory receives notice of the withdrawal or revocation of the accreditation, or
(ii) until the effective date of any action taken by the Secretary under subsection (i).
(D) Evaluations.—The Secretary shall evaluate annually the performance of each approved accreditation body by—
(i) inspecting under subsection (g) a sufficient number of the laboratories accredited by such body to allow a reasonable estimate of the performance of such body, and
(ii) such other means as the Secretary determines appropriate.
(f) Standards.—
(1) In general.—The Secretary shall issue standards to assure consistent performance by laboratories issued a certificate under this section of valid and reliable laboratory examinations and other procedures. Such standards shall require each laboratory issued a certificate under this section—
(A) to maintain a quality assurance and quality control program adequate and appropriate for the validity and reliability of the laboratory examinations and other procedures of the laboratory and to meet requirements relating to the proper collection, transportation, and storage of specimens and the reporting of results,
(B) to maintain records, equipment, and facilities necessary for the proper and effective operation of the laboratory,
(C) in performing and carrying out its laboratory examinations and other procedures, to use only personnel meeting such qualifications as the Secretary may establish for the direction, supervision, and performance of examinations and procedures within the laboratory, which qualifications shall take into consideration competency, training, experience, job performance, and education and which qualifications shall, as appropriate, be different on the basis of the type of examinations and procedures being performed by the laboratory and the risks and consequences of erroneous results associated with such examinations and procedures,
(D) to qualify under a proficiency testing program meeting the standards established by the Secretary under paragraph (3), and
(E) to meet such other requirements as the Secretary determines necessary to assure consistent performance by such laboratories of accurate and reliable laboratory examinations and procedures.
(2) Considerations.—In developing the standards to be issued under paragraph (1), the Secretary shall, within the flexibility provided under subparagraphs (A) through (E) of paragraph (1), take into consideration—
(A) the examinations and procedures performed and the methodologies employed,
(B) the degree of independent judgment involved,
(C) the amount of interpretation involved,
(D) the difficulty of the calculations involved,
(E) the calibration and quality control requirements of the instruments used,
(F) the type of training required to operate the instruments used in the methodology, and
(G) such other factors as the Secretary considers relevant.
(3) Proficiency testing program.—
(A) In general.—The Secretary shall establish standards for the proficiency testing programs for laboratories issued a certificate under this section which are conducted by the Secretary, conducted by an organization approved under subparagraph (C), or conducted by an approved accrediting body. The standards shall require that a laboratory issued a certificate under this section be tested for each examination and procedure conducted within a category of examinations or procedures for which it has received a certificate, except for examinations and procedures for which the Secretary has determined that a proficiency test cannot reasonably be developed. The testing shall be conducted on a quarterly basis, except where the Secretary determines for technical and scientific reasons that a particular examination or procedure may be tested less frequently (but not less often than twice per year).
(B) Criteria.—The standards established under subparagraph (A) shall include uniform criteria for acceptable performance under a proficiency testing program, based on the available technology and the clinical relevance of the laboratory examination or other procedure subject to such program. The criteria shall be established for all examinations and procedures and shall be uniform for each examination and procedure. The standards shall also include a system for grading proficiency testing performance to determine whether a laboratory has performed acceptably for a particular quarter and acceptably for a particular examination or procedure or category of examination or procedure over a period of successive quarters.
(C) Approved proficiency testing programs.—For the purpose of administering proficiency testing programs which meet the standards established under subparagraph (A), the Secretary shall approve a proficiency testing program offered by a private nonprofit organization or a State if the program meets the standards established under subparagraph (A) and the organization or State provides technical assistance to laboratories seeking to qualify under the program. The Secretary shall evaluate each program approved under this subparagraph annually to determine if the program continues to meet the standards established under subparagraph (A) and shall withdraw the approval of any program that no longer meets such standards.
(D) On-site testing.—The Secretary shall perform, or shall direct a program approved under subparagraph (C) to perform, onsite proficiency testing to assure compliance with the requirements of subsection (d)(5). The Secretary shall perform, on an onsite or other basis, proficiency testing to evaluate the performance of a proficiency testing program approved under subparagraph (C) and to assure quality performance by a laboratory.
(E) Training, technical assistance, and enhanced proficiency testing.—The Secretary may, in lieu of or in addition to actions authorized under subsection (h), (i), or (j), require any laboratory which fails to perform acceptably on an individual examination and procedure or a category of examination and procedures—
(i) to undertake training and to obtain the necessary technical assistance to meet the requirements of the proficency [4] testing program,
(ii) to enroll in a program of enhanced proficiency testing, or
(iii) to undertake any combination of the training, technical assistance, or testing described in clauses (i) and (ii).
(F) Testing results.—The Secretary shall establish a system to make the results of the proficiency testing programs subject to the standards established by the Secretary under subparagraph (A) available, on a reasonable basis, upon request of any person. The Secretary shall include with results made available under this subparagraph such explanatory information as may be appropriate to assist in the interpretation of such results.
(4) National standards for quality assurance in cytology services.—
(A) Establishment.—The Secretary shall establish national standards for quality assurance in cytology services designed to assure consistent performance by laboratories of valid and reliable cytological services.
(B) Standards.—The standards established under subparagraph (A) shall include—
(i) the maximum number of cytology slides that any individual may screen in a 24-hour period,
(ii) requirements that a clinical laboratory maintain a record of (I) the number of cytology slides screened during each 24-hour period by each individual who examines cytology slides for the laboratory, and (II) the number of hours devoted during each 24-hour period to screening cytology slides by such individual,
(iii) criteria for requiring rescreening of cytological preparations, such as (I) random rescreening of cytology specimens determined to be in the benign category, (II) focused rescreening of such preparations in high risk groups, and (III) for each abnormal cytological result, rescreening of all prior cytological specimens for the patient, if available,
(iv) periodic confirmation and evaluation of the proficiency of individuals involved in screening or interpreting cytological preparations, including announced and unannounced on-site proficiency testing of such individuals, with such testing to take place, to the extent practicable, under normal working conditions,
(v) procedures for detecting inadequately prepared slides, for assuring that no cytological diagnosis is rendered on such slides, and for notifying referring physicians of such slides,
(vi) requirements that all cytological screening be done on the premises of a laboratory that is certified under this section,
(vii) requirements for the retention of cytology slides by laboratories for such periods of time as the Secretary considers appropriate, and
(viii) standards requiring periodic inspection of cytology services by persons capable of evaluating the quality of cytology services.
(g) Inspections.—
(1) In general.—The Secretary may, on an announced or unannounced basis, enter and inspect, during regular hours of operation, laboratories which have been issued a certificate under this section. In conducting such inspections the Secretary shall have access to all facilities, equipment, materials, records, and information that the Secretary determines have a bearing on whether the laboratory is being operated in accordance with this section. As part of such an inspection the Secretary may copy any such material or require to it[5] be submitted to the Secretary. An inspection under this paragraph may be made only upon presenting identification to the owner, operator, or agent in charge of the laboratory being inspected.
(2) Compliance with requirements and standards.—The Secretary shall conduct inspections of laboratories under paragraph (1) to determine their compliance with the requirements of subsection (d) and the standards issued under subsection (f). Inspections of laboratories not accredited under subsection (e) shall be conducted on a biennial basis or with such other frequency as the Secretary determines to be necessary to assure compliance with such requirements and standards. Inspections of laboratories accredited under subsection (e) shall be conducted on such basis as the Secretary determines is necessary to assure compliance with such requirements and standards.
(h) Intermediate Sanctions.—
(1) In general.—If the Secretary determines that a laboratory which has been issued a certificate under this section no longer substantially meets the requirements for the issuance of a certificate, the Secretary may impose intermediate sanctions in lieu of the actions authorized by subsection (i).
(2) Types of sanctions.—The intermediate sanctions which may be imposed under paragraph (1) shall consist of—
(A) directed plans of correction,
(B) civil money penalties in an amount not to exceed $10,000 for each violation listed in subsection (i)(1) or for each day of substantial noncompliance with the requirements of this section,
(C) payment for the costs of onsite monitoring, or
(D) any combination of the actions described in subparagraphs (A), (B), and (C).
(3) Procedures.—The Secretary shall develop and implement procedures with respect to when and how each of the intermediate sanctions is to be imposed under paragraph (1). Such procedures shall provide for notice to the laboratory and a reasonable opportunity to respond to the proposed sanction and appropriate procedures for appealing determinations relating to the imposition of intermediate sanctions[6]
(i) Suspension, Revocation, and Limitation.—
(1) In general.—Except as provided in paragraph (2), the certificate of a laboratory issued under this section may be suspended, revoked, or limited if the Secretary finds, after reasonable notice and opportunity for hearing to the owner or operator of the laboratory, that such owner or operator or any employee of the laboratory—
(A) has been guilty of misrepresentation in obtaining the certificate,
(B) has performed or represented the laboratory as entitled to perform a laboratory examination or other procedure which is not within a category of laboratory examinations or other procedures authorized in the certificate,
(C) has failed to comply with the requirements of subsection (d) or the standards prescribed by the Secretary under subsection (f),
(D) has failed to comply with reasonable requests of the Secretary for—
(i) any information or materials, or
(ii) work on materials,
that the Secretary concludes is necessary to determine the laboratory's continued eligibility for its certificate or continued compliance with the Secretary's standards under subsection (f),
(E) has refused a reasonable request of the Secretary, or any Federal officer or employee duly designated by the Secretary, for permission to inspect the laboratory and its operations and pertinent records during the hours the laboratory is in operation,
(F) has violated or aided and abetted in the violation of any provisions of this section or of any regulation promulgated thereunder, or
(G) has not complied with an intermediate sanction imposed under subsection (h).
(2) Action before a hearing.—If the Secretary determines that—
(A) the failure of a laboratory to comply with the standards of the Secretary under subsection (f) presents an imminent and serious risk to human health, or
(B) a laboratory has engaged in an action described in subparagraph (D) or (E) of paragraph (1),
the Secretary may suspend or limit the certificate of the laboratory before holding a hearing under paragraph (1) regarding such failure or refusal. The opportunity for a hearing shall be provided no later than 60 days from the effective date of the suspension or limitation. A suspension or limitation under this paragraph shall stay in effect until the decision of the Secretary made after the hearing under paragraph (1).
(3) Ineligibility to own or operate laboratories after revocation.—No person who has owned or operated a laboratory which has had its certificate revoked may, within 2 years of the revocation of the certificate, own or operate a laboratory for which a certificate has been issued under this section. The certificate of a laboratory which has been excluded from participation under the medicare program under title XVIII of the Social Security Act because of actions relating to the quality of the laboratory shall be suspended for the period the laboratory is so excluded.
(4) Improper referrals.—Any laboratory that the Secretary determines intentionally refers its proficiency testing samples to another laboratory for analysis shall have its certificate revoked for at least one year and shall be subject to appropriate fines and penalties as provided for in subsection (h).
(j) Injunctions.—Whenever the Secretary has reason to believe that continuation of any activity by a laboratory would constitute a significant hazard to the public health the Secretary may bring suit in the district court of the United States for the district in which such laboratory is situated to enjoin continuation of such activity. Upon proper showing, a temporary injunction or restraining order against continuation of such activity pending issuance of a final order under this subsection shall be granted without bond by such court.
(k) Judicial Review.—
(1) Petition.—Any laboratory which has had an intermediate sanction imposed under subsection (h) or has had its certificate suspended, revoked, or limited under subsection (i) may, at any time within 60 days after the date the action of the Secretary under subsection (i) or (h) becomes final, file a petition with the United States court of appeals for the circuit wherein the laboratory has its principal place of business for judicial review of such action. As soon as practicable after receipt of the petition, the clerk of the court shall transmit a copy of the petition to the Secretary or other officer designated by the Secretary for that purpose. As soon as practicable after receipt of the copy, the Secretary shall file in the court the record on which the action of the Secretary is based, as provided in section 2112 of title 28, United State Code.
(2) Additional evidence.—If the petitioner applies to the court for leave to adduce additional evidence, and shows to the satisfaction of the court that such additional evidence is material and that there were reasonable grounds for the failure to adduce such evidence in the proceeding before the Secretary, the court may order such additional evidence (and evidence in rebuttal of such additional evidence) to be taken before the Secretary, and to be adduced upon the hearing in such manner and upon such terms and conditions as the court may deem proper. The Secretary may modify the findings of the Secretary as to the facts, or make new findings, by reason of the additional evidence so taken, and the Secretary shall file such modified or new findings, and the recommendations of the Secretary, if any, for the modification or setting aside of his original action, with the return of such additional evidence.
(3) Judgment of court.—Upon the filing of the petition referred to in paragraph (1), the court shall have jurisdiction to affirm the action, or to set it aside in whole or in part, temporarily or permanently. The findings of the Secretary as to the facts, if supported by substantial evidence, shall be conclusive.
(4) Finality of judgment.—The judgment of the court affirming or setting aside, in whole or in part, any such action of the Secretary shall be final, subject to review by the Supreme Court of the United States upon certiorari or certification as provided in section 1254 of title 28, United States Code.
(l) Sanctions.—Any person who intentionally violates any requirement of this section or any regulation promulgated thereunder shall be imprisoned for not more than one year or fined under title 18, United States Code or both, except that if the conviction is for a second or subsequent violation of such a requirement such person shall be imprisoned for not more than 3 years or fined in accordance with title 18, United States Code or both.
(m) Fees.—
(1) Certificate fees.— The Secretary shall require payment of fees for the issuance and renewal of certificates, except that the Secretary shall only require a nominal fee for the issuance and renewal of certificates of waiver.
(2) Additional fees.—The Secretary shall require the payment of fees for inspections of laboratories which are not accredited and for the cost of performing proficiency testing on laboratories which do not participate in proficiency testing programs approved under subsection (f)(3)(C).
(3) Criteria.—
(A) Fees under paragraph (1).—Fees imposed under paragraph (1) shall be sufficient to cover the general costs of administering this section, including evaluating and monitoring proficiency testing programs approved under subsection (f) and accrediting bodies and implementing and monitoring compliance with the requirements of this section.
(B) Fees under paragraph (2).—Fees imposed under paragraph (2) shall be sufficient to cover the cost of the Secretary in carrying out the inspections and proficiency testing described in paragraph (2).
(C) Fees imposed under paragraphs (1) and(2).—Fees imposed under paragraphs (1) and (2) shall vary by group or classification of laboratory, based on such considerations as the Secretary determines are relevant, which may include the dollar volume and scope of the testing being performed by the laboratories.
(n) Information.—On April 1, 1990 and annually thereafter, the Secretary shall compile and make available to physicians and the general public information, based on the previous calendar year, which the Secretary determines is useful in evaluating the performance of a laboratory, including—
(1) a list of laboratories which have been convicted under Federal or State laws relating to fraud and abuse, false billings, or kickbacks,
(2) a list of laboratories—
(A) which have had their certificates revoked, suspended, or limited under subsection (i), or
(B) which have been the subject of a sanction under subsection (l),
together with a statement of the reasons for the revocation, suspension, limitation, or sanction,
(3) a list of laboratories subject to intermediate sanctions under subsection (h) together with a statement of the reasons for the sanctions,
(4) a list of laboratories whose accreditation has been withdrawn or revoked together with a statement of the reasons for the withdrawal or revocation,
(5) a list of laboratories against which the Secretary has taken action under subsection (j) together with a statement of the reasons for such action, and
(6) a list of laboratories which have been excluded from participation under title XVIII or XIX of the Social Security Act.
The information to be compiled under paragraphs (1) through (6) shall be information for the calendar year preceding the date the information is to be made available to the public and shall be accompanied by such explanatory information as may be appropriate to assist in the interpretation of the information compiled under such paragraphs.
(o) Delegation.—In carrying out this section, the Secretary may, pursuant to agreement, use the services or facilities of any Federal or State or local public agency or nonprofit private organization, and may pay therefor in advance or by way of reimbursement, and in such installments, as the Secretary may determine.
(p) State Laws.—
(1) Except as provided in paragraph (2), nothing in this section shall be construed as affecting the power of any State to enact and enforce laws relating to the matters covered by this section to the extent that such laws are not inconsistent with this section or with the regulations issued under this section.
(2) If a State enacts laws relating to matters covered by this section which provide for requirements equal to or more stringent than the requirements of this section or than the regulations issued under this section, the Secretary may exempt clinical laboratories in that State from compliance with this section.
(q) Consultations.—In carrying out this section, the Secretary shall consult with appropriate private organizations and public agencies.
* * * * * * *
Sec. 354.[42 U.S.C. 263b]
(a) Definitions
As used in this section:
(1) Accreditation body
The term “accreditation body” means a body that has been approved by the Secretary under subsection (e)(1)(A) of this section to accredit mammography facilities.
(2) Certificate
The term “certificate” means the certificate described in subsection (b)(1) of this section.
(3) Facility
(A) In general
The term “facility” means a hospital, outpatient department, clinic, radiology practice, or mobile unit, an office of a physician, or other facility as determined by the Secretary, that conducts breast cancer screening or diagnosis through mammography activities. Such term does not include a facility of the Department of Veterans Affairs.
(B) Activities
For the purposes of this section, the activities of a facility include the operation of equipment to produce the mammogram, the processing of the film, the initial interpretation of the mammogram and the viewing conditions for that interpretation. Where procedures such as the film processing, or the interpretation of the mammogram are performed in a location different from where the mammogram is performed, the facility performing the mammogram shall be responsible for meeting the quality standards described in subsection (f) of this section.
(4) Inspection
The term “inspection” means an onsite evaluation of the facility by the Secretary, or State or local agency on behalf of the Secretary.
(5) Mammogram
The term “mammogram” means a radiographic image produced through mammography.
(6) Mammography
The term “mammography” means radiography of the breast.
(7) Survey
The term “survey” means an onsite physics consultation and evaluation performed by a medical physicist as described in subsection (f)(1)(E) of this section.
(8) Review physician
The term “review physician” means a physician as prescribed by the Secretary under subsection (f)(1)(D) of this section who meets such additional requirements as may be established by an accreditation body under subsection (e) of this section and approved by the Secretary to review clinical images under subsection (e)(1)(B)(i) of this section on behalf of the accreditation body.
(b) Certificate requirement
(1) Certificate
No facility may conduct an examination or procedure described in paragraph (2) involving mammography after October 1, 1994, unless the facility obtains—
(A) a certificate—
(i) that is issued, and, if applicable, renewed, by the Secretary in accordance with subsection (c)(1) of this section;
(ii) that is applicable to the examination or procedure to be conducted; and
(iii) that is displayed prominently in such facility; or
(B) a provisional certificate—
(i) that is issued by the Secretary in accordance with subsection (c)(2) of this section;
(ii) that is applicable to the examination or procedure to be conducted; and
(iii) that is displayed prominently in such facility.
The reference to a certificate in this section includes a provisional certificate.
(2) Examination or procedure
A facility shall obtain a certificate in order to—
(A) operate radiological equipment that is used to image the breast;
(B) provide for the interpretation of a mammogram produced by such equipment at the facility or under arrangements with a qualified individual at a facility different from where the mammography examination is performed; and
(C) provide for the processing of film produced by such equipment at the facility or under arrangements with a qualified individual at a facility different from where the mammography examination is performed.
(c) Issuance and renewal of certificates
(1) In general
The Secretary may issue or renew a certificate for a facility if the person or agent described in subsection (d)(1)(A) of this section meets the applicable requirements of subsection (d)(1) of this section with respect to the facility. The Secretary may issue or renew a certificate under this paragraph for not more than 3 years.
(2) Provisional certificate
The Secretary may issue a provisional certificate for an entity to enable the entity to qualify as a facility. The applicant for a provisional certificate shall meet the requirements of subsection (d)(1) of this section, except providing information required by clauses (iii) and (iv) of subsection (d)(1)(A) of this section. A provisional certificate may be in effect no longer than 6 months from the date it is issued, except that it may be extended once for a period of not more than 90 days if the owner, lessor, or agent of the facility demonstrates to the Secretary that without such extension access to mammography in the geographic area served by the facility would be significantly reduced and if the owner, lessor, or agent of the facility will describe in a report to the Secretary steps that will be taken to qualify the facility for certification under subsection (b)(1) of this section.
(d) Application for certificate
(1) Submission
The Secretary may issue or renew a certificate for a facility if—
(A) the person who owns or leases the facility or an authorized agent of the person, submits to the Secretary, in such form and manner as the Secretary shall prescribe, an application that contains at a minimum—
(i) a description of the manufacturer, model, and type of each x-ray machine, image receptor, and processor operated in the performance of mammography by the facility;
(ii) a description of the procedures currently used to provide mammography at the facility, including—
(I) the types of procedures performed and the number of such procedures performed in the prior 12 months;
(II) the methodologies for mammography; and
(III) the names and qualifications (educational background, training, and experience) of the personnel performing mammography and the physicians reading and interpreting the results from the procedures;
(iii) proof of on-site survey by a qualified medical physicist as described in subsection (f)(1)(E) of this section; and
(iv) proof of accreditation in such manner as the Secretary shall prescribe; and
(B) the person or agent submits to the Secretary—
(i) a satisfactory assurance that the facility will be operated in accordance with standards established by the Secretary under subsection (f) of this section to assure the safety and accuracy of mammography;
(ii) a satisfactory assurance that the facility will—
(I) permit inspections under subsection (g) of this section;
(II) make such records and information available, and submit such reports, to the Secretary as the Secretary may require; and
(III) update the information submitted under subparagraph (A) or assurances submitted under this subparagraph on a timely basis as required by the Secretary; and
(iii) such other information as the Secretary may require.
An applicant shall not be required to provide in an application under subparagraph (A) any information which the applicant has supplied to the accreditation body which accredited the applicant, except as required by the Secretary.
(2) Appeal
If the Secretary denies an application for the certification of a facility submitted under paragraph (1)(A), the Secretary shall provide the owner or lessor of the facility or the agent of the owner or lessor who submitted such application—
(A) a statement of the grounds on which the denial is based, and
(B) an opportunity for an appeal in accordance with the procedures set forth in regulations of the Secretary published at part 498 of title 42 Code of Federal Regulations.
(3) Effect of denial
If the application for the certification of a facility is denied, the facility may not operate unless the denial of the application is overturned at the conclusion of the administrative appeals process provided in the regulations referred to in paragraph (2)(B).
(e) Accreditation
(1) Approval of accreditation bodies
(A) In general
The Secretary may approve a private nonprofit organization or State agency to accredit facilities for purposes of subsection (d)(1)(A)(iv) of this section if the accreditation body meets the standards for accreditation established by the Secretary as described in subparagraph (B) and provides the assurances required by subparagraph (C).
(B) Standards
The Secretary shall establish standards for accreditation bodies, including—
(i) standards that require an accreditation body to perform—
(I) a review of clinical images from each facility accredited by such body not less often than every 3 years which review will be made by qualified practicing physicians; and
(II) a review of a random sample of clinical images from such facilities in each 3-year period beginning October 1, 1994, which review will be made by qualified review physicians;
(ii) standards that prohibit individuals conducting the reviews described in clause (i) from maintaining any relationship to the facility undergoing review which would constitute a conflict of interest;
(iii) standards that limit the imposition of fees for accreditation to reasonable amounts;
(iv) standards that require as a condition of accreditation that each facility undergo a survey at least annually by a medical physicist as described in subsection (f)(1)(E) of this section to ensure that the facility meets the standards described in subparagraphs (A) and (B) of subsection (f)(1) of this section;
(v) standards that require monitoring and evaluation of such survey, as prescribed by the Secretary;
(vi) standards that are equal to standards established under subsection (f) of this section which are relevant to accreditation as determined by the Secretary; and
(vii) such additional standards as the Secretary may require.
(C) Assurances
The accrediting body shall provide the Secretary satisfactory assurances that the body will—
(i) comply with the standards as described in subparagraph (B);
(ii) comply with the requirements described in paragraph (4);
(iii) submit to the Secretary the name of any facility for which the accreditation body denies, suspends, or revokes accreditation;
(iv) notify the Secretary in a timely manner before the accreditation body changes the standards of the body;
(v) notify each facility accredited by the accreditation body if the Secretary withdraws approval of the accreditation body under paragraph (2) in a timely manner; and
(vi) provide such other additional information as the Secretary may require.
(D) Regulations
Not later than 9 months after October 27, 1992, the Secretary shall promulgate regulations under which the Secretary may approve an accreditation body.
(2) Withdrawal of approval
(A) In general
The Secretary shall promulgate regulations under which the Secretary may withdraw the approval of an accreditation body if the Secretary determines that the accreditation body does not meet the standards under subparagraph (B) of paragraph (1), the requirements of clauses (i) through (vi) of subparagraph (C) of paragraph (1), or the requirements of paragraph (4).
(B) Effect of withdrawal
If the Secretary withdraws the approval of an accreditation body under subparagraph (A), the certificate of any facility accredited by the body shall continue in effect until the expiration of a reasonable period, as determined by the Secretary, for such facility to obtain another accreditation.
(3) Accreditation
To be accredited by an approved accreditation body a facility shall meet—
(A) the standards described in paragraph (1)(B) which the Secretary determines are applicable to the facility, and
(B) such other standards which the accreditation body may require.
(4) Compliance
To ensure that facilities accredited by an accreditation body will continue to meet the standards of the accreditation body, the accreditation body shall—
(A) make onsite visits on an annual basis of a sufficient number of the facilities accredited by the body to allow a reasonable estimate of the performance of the body; and
(B) take such additional measures as the Secretary determines to be appropriate.
Visits made under subparagraph (A) shall be made after providing such notice as the Secretary may require.
(5) Revocation of accreditation
If an accreditation body revokes the accreditation of a facility, the certificate of the facility shall continue in effect until such time as may be determined by the Secretary.
(6) Evaluation and report
(A) Evaluation
The Secretary shall evaluate annually the performance of each approved accreditation body by—
(i) inspecting under subsection (g)(2) of this section a sufficient number of the facilities accredited by the body to allow a reasonable estimate of the performance of the body; and
(ii) such additional means as the Secretary determines to be appropriate.
(B) Report
The Secretary shall annually prepare and submit to the Committee on Labor and Human Resources of the Senate and the Committee on Energy and Commerce of the House of Representatives a report that describes the results of the evaluation conducted in accordance with subparagraph (A).
(f) Quality standards
(1) In general
The standards referred to in subsection (d)(1)(B)(i) of this section are standards established by the Secretary which include—
(A) standards that require establishment and maintenance of a quality assurance and quality control program at each facility that is adequate and appropriate to ensure the reliability, clarity, and accuracy of interpretation of mammograms and standards for appropriate radiation dose;
(B) standards that require use of radiological equipment specifically designed for mammography, including radiologic standards and standards for other equipment and materials used in conjunction with such equipment;
(C) a requirement that personnel who perform mammography—
(i)(I) be licensed by a State to perform radiological procedures; or
(II) be certified as qualified to perform radiological procedures by an organization described in paragraph (2)(A); and
(ii) during the 2-year period beginning October 1, 1994, meet training standards for personnel who perform mammography or meet experience requirements which shall at a minimum include 1 year of experience in the performance of mammography; and
(iii) upon the expiration of such 2-year period meet minimum training standards for personnel who perform mammograms;
(D) a requirement that mammograms be interpreted by a physician who is certified as qualified to interpret radiological procedures, including mammography—
(i)(I) by a board described in paragraph (2)(B); or
(II) by a program that complies with the standards described in paragraph (2)(C); and
(ii) who meets training and continuing medical education requirements as established by the Secretary;
(E) a requirement that individuals who survey mammography facilities be medical physicists—
(i) licensed or approved by a State to perform such surveys, reviews, or inspections for mammography facilities;
(ii) certified in diagnostic radiological physics or certified as qualified to perform such surveys by a board as described in paragraph (2)(D); or
(iii) in the first 5 years after October 27, 1992, who meet other criteria established by the Secretary which are comparable to the criteria described in clause (i) or (ii);
(F) a requirement that a medical physicist who is qualified in mammography as described in subparagraph (E) survey mammography equipment and oversee quality assurance practices at each facility;
(G) a requirement that—
(i) a facility that performs any mammogram maintain the mammogram in the permanent medical records of the patient—
(I) except as provided in subclause (II), maintain the mammogram in the permanent medical records of the patient for a period of not less than 5 years, or not less than 10 years if no subsequent mammograms of such patient are performed at the facility, or longer if mandated by State law; and
(II) upon the request of or on behalf of the patient, transfer the mammogram to a medical institution, to a physician of the patient, or to the patient directly; and
whichever is longer; and
(ii)(I) a facility must assure the preparation of a written report of the results of any mammography examination signed by the interpreting physician;
(II) such written report shall be provided to the patient's physicians (if any);
(III) if such a physician is not available or if there is no such physician, the written report shall be sent directly to the patient; and
(IV) whether or not such a physician is available or there is no such physician, a summary of the written report shall be sent directly to the patient in terms easily understood by a lay person; and
(H) standards relating to special techniques for mammography of patients with breast implants.
Subparagraph (G) shall not be construed to limit a patient's access to the patient's medical records.
(2) Certification of personnel
The Secretary shall by regulation—
(A) specify organizations eligible to certify individuals to perform radiological procedures as required by paragraph (1)(C);
(B) specify boards eligible to certify physicians to interpret radiological procedures, including mammography, as required by paragraph (1)(D);
(C) establish standards for a program to certify physicians described in paragraph (1)(D); and
(D) specify boards eligible to certify medical physicists who are qualified to survey mammography equipment and to oversee quality assurance practices at mammography facilities.
(g) Inspections
(1) Annual inspections
(A) In general
The Secretary may enter and inspect facilities to determine compliance with the certification requirements under subsection (b) of this section and the standards established under subsection (f) of this section. The Secretary shall, if feasible, delegate to a State or local agency the authority to make such inspections.
(B) Identification
The Secretary, or State agency acting on behalf of the Secretary, may conduct inspections only on presenting identification to the owner, operator, or agent in charge of the facility to be inspected.
(C) Scope of inspection
In conducting inspections, the Secretary or State or local agency acting on behalf of the Secretary—
(i) shall have access to all equipment, materials, records, and information that the Secretary or State or local agency considers necessary to determine whether the facility is being operated in accordance with this section; and
(ii) may copy, or require the facility to submit to the Secretary or the State or local agency, any of the materials, records, or information.
(D) Qualifications of inspectors
Qualified individuals, as determined by the Secretary, shall conduct all inspections. The Secretary may request that a State agency acting on behalf of the Secretary designate a qualified officer or employee to conduct the inspections, or designate a qualified Federal officer or employee to conduct inspections. The Secretary shall establish minimum qualifications and appropriate training for inspectors and criteria for certification of inspectors in order to inspect facilities for compliance with subsection (f) of this section.
(E) Frequency
The Secretary or State agency acting on behalf of the Secretary shall conduct inspections under this paragraph of each facility not less often than annually subject to paragraph (6).
(F) Records and annual reports
The Secretary or a State or local agency acting on behalf of the Secretary which is responsible for inspecting mammography facilities shall maintain records of annual inspections required under this paragraph for a period as prescribed by the Secretary. Such a State or local agency shall annually prepare and submit to the Secretary a report concerning the inspections carried out under this paragraph. Such reports shall include a description of the facilities inspected and the results of such inspections.
(2) Inspection of accredited facilities
The Secretary shall inspect annually a sufficient number of the facilities accredited by an accreditation body to provide the Secretary with a reasonable estimate of the performance of such body.
(3) Inspection of facilities inspected by State or local agencies
The Secretary shall inspect annually facilities inspected by State agencies acting on behalf of the Secretary to assure a reasonable performance by such State or local agencies.
(4) Timing
The Secretary, or State or local agency, may conduct inspections under paragraphs (1), (2), and (3), during regular business hours or at a mutually agreeable time and after providing such notice as the Secretary may prescribe, except that the Secretary may waive such requirements if the continued performance of mammography at such facility threatens the public health.
(5) Limited reinspection
Nothing in this section limits the authority of the Secretary to conduct limited reinspections of facilities found not to be in compliance with this section.
6) Demonstration program
(A) In general
The Secretary may establish a demonstration program under which inspections under paragraph (1) of selected facilities are conducted less frequently by the Secretary (or as applicable, by State or local agencies acting on behalf of the Secretary) than the interval specified in subparagraph (E) of such paragraph.
(B) Requirements
Any demonstration program under subparagraph (A) shall be carried out in accordance with the following:
(i) The program may not be implemented before April 1, 2001. Preparations for the program may be carried out prior to such date.
(ii) In carrying out the program, the Secretary may not select a facility for inclusion in the program unless the facility is substantially free of incidents of noncompliance with the standards under subsection (f) of this section. The Secretary may at any time provide that a facility will no longer be included in the program.
(iii) The number of facilities selected for inclusion in the program shall be sufficient to provide a statistically significant sample, subject to compliance with clause (ii).
(iv) Facilities that are selected for inclusion in the program shall be inspected at such intervals as the Secretary determines will reasonably ensure that the facilities are maintaining compliance with such standards.
(h) Sanctions
(1) In general
In order to promote voluntary compliance with this section, the Secretary may, in lieu of taking the actions authorized by subsection (i) of this section, impose one or more of the following sanctions;
(A) Directed plans of correction which afford a facility an opportunity to correct violations in a timely manner.
(B) Payment for the cost of onsite monitoring.
(2) Patient Information
If the Secretary determines that the quality of mammography performed by a facility (whether or not certified pursuant to subsection (c) of this section) was so inconsistent with the quality standards established pursuant to subsection (f) of this section as to present a significant risk to individual or public health, the Secretary may require such facility to notify patients who received mammograms at such facility, and their referring physicians, of the deficiencies presenting such risk, the potential harm resulting, appropriate remedial measures, and such other relevant information as the Secretary may require.
(3) Civil money penalties
The Secretary may assess civil money penalties in an amount not to exceed $10,000 for—
(A) failure to obtain a certificate as required by subsection (b) of this section,
(B) each failure by a facility to substantially comply with, or each day on which a facility fails to substantially comply with, the standards established under subsection (f) of this section or the requirements described in subclauses (I) through (III) of subsection (d)(1)(B)(ii) of this section, and
(C) each failure to notify a patient of risk as required by the Secretary pursuant to paragraph (2), and
(D) each violation, or for each aiding and abetting in a violation of, any provision of, or regulation promulgated under, this section by an owner, operator, or any employee of a facility required to have a certificate.
(4) Procedures
The Secretary shall develop and implement procedures with respect to when and how each of the sanctions is to be imposed under paragraphs (1) through (4). Such procedures shall provide for notice to the owner or operator of the facility and a reasonable opportunity for the owner or operator to respond to the proposed sanctions and appropriate procedures for appealing determinations relating to the imposition of sanctions.
(i) Suspension and revocation
(1) In general
The certificate of a facility issued under subsection (c) of this section may be suspended or revoked if the Secretary finds, after providing, except as provided in paragraph (2), reasonable notice and an opportunity for a hearing to the owner or operator of the facility, that the owner, operator, or any employee of the facility—
(A) has been guilty of misrepresentation in obtaining the certificate;
(B) has failed to comply with the requirements of subsection (d)(1)(B)(ii)(III) of this section or the standards established by the Secretary under subsection (f) of this section;
(C) has failed to comply with reasonable requests of the Secretary (or of an accreditation body approved pursuant to subsection (e) of this section) for any record, information, report, or material that the Secretary (or such accreditation body or State carrying out certification program requirements pursuant to subsection (q) of this section) concludes is necessary to determine the continued eligibility of the facility for a certificate or continued compliance with the standards established under subsection (f) of this section;
(D) has refused a reasonable request of the Secretary, any Federal officer or employee duly designated by the Secretary, or any State or local officer or employee duly designated by the State or local agency, for permission to inspect the facility or the operations and pertinent records of the facility in accordance with subsection (g) of this section;
(E) has violated or aided and abetted in the violation of any provision of, or regulation promulgated under, this section; or
(F) has failed to comply with a sanction imposed under subsection (h) of this section.
(2) Action before a hearing
(A) In general
The Secretary may suspend the certificate of the facility before holding a hearing required by paragraph (1) if the Secretary has reason to believe that the circumstance of the case will support one or more of the findings described in paragraph (1) and determines that—
(i) the failure or violation was intentional; or
(ii) the failure or violation presents a serious risk to human health.
(B) Hearing
If the Secretary suspends a certificate under subparagraph (A), the Secretary shall provide an opportunity for a hearing to the owner or operator of the facility not later than 60 days from the effective date of the suspension. The suspension shall remain in effect until the decision of the Secretary made after the hearing.
(3) Ineligibility to own or operate facilities after revocation
If the Secretary revokes the certificate of a facility on the basis of an act described in paragraph (1), no person who owned or operated the facility at the time of the act may, within 2 years of the revocation of the certificate, own or operate a facility that requires a certificate under this section.
(j) Injunctions
If the Secretary determines that—
(1) continuation of any activity related to the provision of mammography by a facility would constitute a serious risk to human health, the Secretary may bring suit in the district court of the United States for the district in which the facility is situated to enjoin continuation of the activity; and
(2) a facility is operating without a certificate as required by subsection (b) of this section, the Secretary may bring suit in the district court of the United States for the district in which the facility is situated to enjoin the operation of the facility.
Upon a proper showing, the district court shall grant a temporary injunction or restraining order against continuation of the activity or against operation of a facility, as the case may be, without requiring the Secretary to post a bond, pending issuance of a final order under this subsection.
(k) Judicial review
(1) Petition
If the Secretary imposes a sanction on a facility under subsection (h) of this section or suspends or revokes the certificate of a facility under subsection (i) of this section, the owner or operator of the facility may, not later than 60 days after the date the action of the Secretary becomes final, file a petition with the United States court of appeals for the circuit in which the facility is situated for judicial review of the action. As soon as practicable after receipt of the petition, the clerk of the court shall transmit a copy of the petition to the Secretary or other officer designated by the Secretary. As soon as practicable after receipt of the copy, the Secretary shall file in the court the record on which the action of the Secretary is based, as provided in section 2112 of title 28.
(2) Additional evidence
If the petitioner applies to the court for leave to adduce additional evidence, and shows to the satisfaction of the court that the additional evidence is material and that there were reasonable grounds for the failure to adduce such evidence in the proceeding before the Secretary, the court may order the additional evidence (and evidence in rebuttal of the additional evidence) to be taken before the Secretary, and to be adduced upon the hearing in such manner and upon such terms and conditions as the court may determine to be proper. The Secretary may modify the findings of the Secretary as to the facts, or make new findings, by reason of the additional evidence so taken, and the Secretary shall file the modified or new findings, and the recommendations of the Secretary, if any, for the modification or setting aside of the original action of the Secretary with the return of the additional evidence.
(3) Judgment of court
Upon the filing of the petition referred to in paragraph (1), the court shall have jurisdiction to affirm the action, or to set the action aside in whole or in part, temporarily or permanently. The findings of the Secretary as to the facts, if supported by substantial evidence, shall be conclusive.
(4) Finality of judgment
The judgment of the court affirming or setting aside, in whole or in part, any action of the Secretary shall be final, subject to review by the Supreme Court of the United States upon certiorari or certification, as provided in section 1254 of title 28.
(l) Information
(1) In general
Not later than October 1, 1996, and annually thereafter, the Secretary shall compile and make available to physicians and the general public information that the Secretary determines is useful in evaluating the performance of facilities, including a list of facilities—
(A) that have been convicted under Federal or State laws relating to fraud and abuse, false billings, or kickbacks;
(B) that have been subject to sanctions under subsection (h) of this section, together with a statement of the reasons for the sanctions;
(C) that have had certificates revoked or suspended under subsection (i) of this section, together with a statement of the reasons for the revocation or suspension;
(D) against which the Secretary has taken action under subsection (j) of this section, together with a statement of the reasons for the action;
(E) whose accreditation has been revoked, together with a statement of the reasons of the revocation;
(F) against which a State has taken adverse action; and
(G) that meets such other measures of performance as the Secretary may develop.
(2) Date
The information to be compiled under paragraph (1) shall be information for the calendar year preceding the date the information is to be made available to the public.
(3) Explanatory information
The information to be compiled under paragraph (1) shall be accompanied by such explanatory information as may be appropriate to assist in the interpretation of the information compiled under such paragraph.
(m) State laws
Nothing in this section shall be construed to limit the authority of any State to enact and enforce laws relating to the matters covered by this section that are at least as stringent as this section or the regulations issued under this section.
(n) National Advisory Committee
(1) Establishment
In carrying out this section, the Secretary shall establish an advisory committee to be known as the National Mammography Quality Assurance Advisory Committee (hereafter in this subsection referred to as the “Advisory Committee”).
(2) Composition
The Advisory Committee shall be composed of not fewer than 13, nor more than 19 individuals, who are not officers or employees of the Federal Government. The Secretary shall make appointments to the Advisory Committee from among—
(A) physicians,
(B) practitioners, and
(C) other health professionals,
whose clinical practice, research specialization, or professional expertise include a significant focus on mammography. The Secretary shall appoint at least 4 individuals from among national breast cancer or consumer health organizations with expertise in mammography and at least 2 practicing physicians who provide mammography services.
(3) Functions and duties
The Advisory Committee shall—
(A) advise the Secretary on appropriate quality standards and regulations for mammography facilities;
(B) advise the Secretary on appropriate standards and regulations for accreditation bodies;
(C) advise the Secretary in the development of regulations with respect to sanctions;
(D) assist in developing procedures for monitoring compliance with standards under subsection (f) of this section;
(E) make recommendations and assist in the establishment of a mechanism to investigate consumer complaints;
(F) report on new developments concerning breast imaging that should be considered in the oversight of mammography facilities;
(G) determine whether there exists a shortage of mammography facilities in rural and health professional shortage areas and determine the effects of personnel or other requirements of subsection (f) of this section on access to the services of such facilities in such areas;
(H) determine whether there will exist a sufficient number of medical physicists after October 1, 1999, to assure compliance with the requirements of subsection (f)(1)(E) of this section;
(I) determine the costs and benefits of compliance with the requirements of this section (including the requirements of regulations promulgated under this section); and
(J) perform other activities that the Secretary may require.
The Advisory Committee shall report the findings made under subparagraphs (G) and (I) to the Secretary and the Congress no later than October 1, 1993.
(4) Meetings
The Advisory Committee shall meet not less than quarterly for the first 3 years of the program and thereafter, at least biannually.
(5) Chairperson
The Secretary shall appoint a chairperson of the Advisory Committee.
(o) Consultations
In carrying out this section, the Secretary shall consult with appropriate Federal agencies within the Department of Health and Human Services for the purposes of developing standards, regulations, evaluations, and procedures for compliance and oversight.
(p) Breast cancer screening surveillance research grants
(1) Research
(A) Grants
The Secretary shall award grants to such entities as the Secretary may determine to be appropriate to establish surveillance systems in selected geographic areas to provide data to evaluate the functioning and effectiveness of breast cancer screening programs in the United States, including assessments of participation rates in screening mammography, diagnostic procedures, incidence of breast cancer, mode of detection (mammography screening or other methods), outcome and follow up information, and such related epidemiologic analyses that may improve early cancer detection and contribute to reduction in breast cancer mortality. Grants may be awarded for further research on breast cancer surveillance systems upon the Secretary's review of the evaluation of the program.
(B) Use of funds
Grants awarded under subparagraph (A) may be used—
(i) to study—
(I) methods to link mammography and clinical breast examination records with population-based cancer registry data;
(II) methods to provide diagnostic outcome data, or facilitate the communication of diagnostic outcome data, to radiology facilities for purposes of evaluating patterns of mammography interpretation; and
(III) mechanisms for limiting access and maintaining confidentiality of all stored data; and
(ii) to conduct pilot testing of the methods and mechanisms described in subclauses (I), (II), and (III) of clause (i) on a limited basis.
(C) Grant application
To be eligible to receive funds under this paragraph, an entity shall submit an application to the Secretary at such time, in such manner, and containing such information as the Secretary may require.
(D) Report
A recipient of a grant under this paragraph shall submit a report to the Secretary containing the results of the study and testing conducted under clauses (i) and (ii) of subparagraph (B), along with recommendations for methods of establishing a breast cancer screening surveillance system.
(2) Establishment
The Secretary shall establish a breast cancer screening surveillance system based on the recommendations contained in the report described in paragraph (1)(D).
(3) Standards and procedures
The Secretary shall establish standards and procedures for the operation of the breast cancer screening surveillance system, including procedures to maintain confidentiality of patient records.
(4) Information
The Secretary shall recruit facilities to provide to the breast cancer screening surveillance system relevant data that could help in the research of the causes, characteristics, and prevalence of, and potential treatments for, breast cancer and benign breast conditions, if the information may be disclosed under section 552 of title 5.
(q) State program
(1) In general
The Secretary may, upon application, authorize a State—
(A) to carry out, subject to paragraph (2), the certification program requirements under subsections (b), (c), (d), (g)(1), (h), (i), and (j) of this section (including the requirements under regulations promulgated pursuant to such subsections), and
(B) to implement the standards established by the Secretary under subsection (f) of this section,
with respect to mammography facilities operating within the State.
(2) Approval
The Secretary may approve an application under paragraph (1) if the Secretary determines that—
(A) the State has enacted laws and issued regulations relating to mammography facilities which are the requirements of this section (including the requirements under regulations promulgated pursuant to such subsections), and
(B) the State has provided satisfactory assurances that the State—
(i) has the legal authority and qualified personnel necessary to enforce the requirements of and the regulations promulgated pursuant to this section (including the requirements under regulations promulgated pursuant to such subsections),
(ii) will devote adequate funds to the administration and enforcement of such requirements, and
(iii) will provide the Secretary with such information and reports as the Secretary may require.
(3) Authority of Secretary
In a State with an approved application—
(A) the Secretary shall carry out the Secretary's functions under subsections (e) and (f) of this section;
(B) the Secretary may take action under subsections (h), (i), and (j) of this section; and
(C) the Secretary shall conduct oversight functions under subsections (g)(2) and (g)(3) of this section.
(4) Withdrawal of approval
(A) In general
The Secretary may, after providing notice and opportunity for corrective action, withdraw the approval of a State's authority under paragraph (1) if the Secretary determines that the State does not meet the requirements of such paragraph. The Secretary shall promulgate regulations for the implementation of this subparagraph.
(B) Effect of withdrawal
If the Secretary withdraws the approval of a State under subparagraph (A), the certificate of any facility accredited by the State shall continue in effect until the expiration of a reasonable period, as determined by the Secretary, for such facility to obtain certification by the Secretary.
(r) Funding
(1) Fees
(A) In general
The Secretary shall, in accordance with this paragraph assess and collect fees from persons described in subsection (d)(1)(A) of this section (other than persons who are governmental entities, as determined by the Secretary) to cover the costs of inspections conducted under subsection (g)(1) of this section by the Secretary or a State acting under a delegation under subparagraph (A) of such subsection. Fees may be assessed and collected under this paragraph only in such manner as would result in an aggregate amount of fees collected during any fiscal year which equals the aggregate amount of costs for such fiscal year for inspections of facilities of such persons under subsection (g)(1) of this section. A person's liability for fees shall be reasonably based on the proportion of the inspection costs which relate to such person.
(B) Deposit and appropriations
(i) Deposit and availability
Fees collected under subparagraph (A) shall be deposited as an offsetting collection to the appropriations for the Department of Health and Human Services as provided in appropriation Acts and shall remain available without fiscal year limitation.
(ii) Appropriations
Fees collected under subparagraph (A) shall be collected and available only to the extent provided in advance in appropriation Acts.
(2) Authorization of appropriations
There are authorized to be appropriated to carry out this section—
(A) to award research grants under subsection (p) of this section, such sums as may be necessary for each of the fiscal years 1993 through 2002; and
(B) for the Secretary to carry out other activities which are not supported by fees authorized and collected under paragraph (1), such sums as may be necessary for fiscal years 1993 through 2002.
* * * * * * *
Sec. 371.[42 U.S.C. 273]
(a)(1) The Secretary may make grants for the planning of qualified organ procurement organizations described in subsection (b).
(2) The Secretary may make grants for the establishment, initial operation, consolidation, and expansion of qualified organ procurement organizations described in subsection (b).
(3) The Secretary may make grants to, and enter into contracts with, qualified organ procurement organizations described in subsection (b) and other nonprofit private entities for the purpose of carrying out special projects designed to increase the number of organ donors.
(b)(1) A qualified organ procurement organization for which grants may be made under subsection (a) is an organization which, as determined by the Secretary, will carry out the functions described in paragraph (2) and—
(A) is a nonprofit entity,
(B) has accounting and other fiscal procedures (as specified by the Secretary) necessary to assure the fiscal stability of the organization,
(C) has an agreement with the Secretary to be reimbursed under title XVIII of the Social Security Act for the procurement of kidneys,
(D) notwithstanding any other provision of law, has met the other requirements of this section and has been certified or recertified by the Secretary within the previous 4-year period as meeting the performance standards to be a qualified organ procurement organization through a process that either—
(i) granted certification or recertification within such 4-year period with such certification or recertification in effect as of January 1, 2000, and remaining in effect through the earlier of—
(I) January 1, 2002; or
(II) the completion of recertification under the requirements of clause (ii); or
(ii) is defined through regulations that are promulgated by the Secretary by not later than January 1, 2002, that—
(I) require recertifications of qualified organ procurement organizations not more frequently than once every 4 years;
(II) rely on outcome and process performance measures that are based on empirical evidence, obtained through reasonable efforts, of organ donor potential and other related factors in each service area of qualified organ procurement organizations;
(III) use multiple outcome measures as part of the certification process; and
(IV) provide for a qualified organ procurement organization to appeal a decertification to the Secretary on substantive and procedural grounds;[7]
(E) has procedures to obtain payment for non-renal organs provided to transplant centers,
(F) has a defined service area that is of sufficient size to assure maximum effectiveness in the procurement and equitable distribution of organs, and that either includes an entire metropolitan statistical area (as specified by the Director of the Office of Management and Budget) or does not include any part of the area,
(G) has a director and such other staff, including the organ donation coordinators and organ procurement specialists necessary to effectively obtain organs from donors in its service area, and
(H) has a board of directors or an advisory board which—
(i) is composed of—
(I) members who represent hospital administrators, intensive care or emergency room personnel, tissue banks, and voluntary health associations in its service area,
(II) members who represent the public residing in such area,
(III) a physician with knowledge, experience, or skill in the field of histocompatability or an individual with a doctorate degree in a biological science with knowledge, experience, or skill in the field of histocompatibility,
(IV) a physician with knowledge or skill in the field of neurology, and
(V) from each transplant center in its service area which has arrangements described in paragraph (2)(G) with the organization, a member who is a surgeon who has practicing privileges in such center and who performs organ transplant surgery,
(ii) has the authority to recommend policies for the procurement of organs and the other functions described in paragraph (2), and
(iii) has no authority over any other activity of the organization.
(2)(A) Not later than 90 days after the date of the enactment of this paragraph, the Secretary shall publish in the Federal Register a notice of proposed rulemaking to establish criteria for determining whether an entity meets the requirement established in paragraph (1)(E).
(B) Not later than 1 year after the date of enactment of this paragraph, the Secretary shall publish in the Federal Register a final rule to establish the criteria described in subparagraph (A).
(3) [Stricken.]
(c) Pancreata procured by an organ procurement organization and used for islet cell transplantation or research shall be counted for purposes of certification or recertification under subsection (b).
Sec. 372.[42 U.S.C. 274]
(a) The Secretary shall by contract provide for the establishment and operation of an Organ Procurement and Transplantation Network which meets the requirements of subsection (b). The amount provided under such contract in any fiscal year may not exceed $2,000,000. Funds for such contracts shall be made available from funds available to the Public Health Service from appropriations for fiscal years beginning after fiscal year 1984.
(b)(1) The Organ Procurement and Transplantation Network shall carry out the functions described in paragraph (2) and shall—
(A) be a private nonprofit entity that has an expertise in organ procurement and transplantation, and
(B) have a board of directors—
(i) that includes representatives of organ procurement organizations (including organizations that have received grants under section 371), transplant centers, voluntary health associations, and the general public; and
(ii) that shall establish an executive committee and other committees, whose chairpersons shall be selected to ensure continuity of leadership for the board.
(2) The Organ Procurement and Transplantation Network shall—
(A) establish in one location or through regional centers—
(i) a national list of individuals who need organs, and
(ii) a national system, through the use of computers and in accordance with established medical criteria, to match organs and individuals included in the list, especially individuals whose immune system makes it difficult for them to receive organs,
(B) establish membership criteria and medical criteria for allocating organs and provide to members of the public an opportunity to comment with respect to such criteria,
(C) maintain a twenty-four-hour telephone service to facilitate matching organs with individuals included in the list,
(D) assist organ procurement organizations in the nationwide distribution of organs equitably among transplant patients,
(E) adopt and use standards of quality for the acquisition and transportation of donated organs, including standards for preventing the acquisition of organs that are infected with the etiologic agent for acquired immune deficiency syndrome,
(F) prepare and distribute, on a regionalized basis (and, to the extent practicable, among regions or on a national basis), samples of blood sera from individuals who are included on the list and whose immune system makes it difficult for them to receive organs, in order to facilitate matching the compatibility of such individuals with organ donors,
(G) coordinate, as appropriate, the transportation of organs from organ procurement organizations to transplant centers,
(H) provide information to physicians and other health professionals regarding organ donation,
(I) collect, analyze, and publish data concerning organ donation and transplants,
(J) carry out studies and demonstration projects for the purpose of improving procedures for organ procurement and allocation, and
(K) work actively to increase the supply of donated organs.
(L) submit to the Secretary an annual report containing information on the comparative costs and patient outcomes at each transplant center affiliated with the organ procurement and transplantation network.
(M) recognize the differences in health and in organ transplantation issues between children and adults throughout the system and adopt criteria, polices, and procedures that address the unique health care needs of children,
(N) carry out studies and demonstration projects for the purpose of improving procedures for organ donation procurement and allocation, including but not limited to projects to examine and attempt to increase transplantation among populations with special needs, including children and individuals who are members of racial or ethnic minority groups, and among populations with limited access to transportation, and
(O) provide that for purposes of this paragraph, the term “children” refers to individuals who are under the age of 18.
(c) The Secretary shall establish procedures for—
(1) receiving from interested persons critical comments relating to the manner in which the Organ Procurement and Transplantation Network is carrying out the duties of the Network under subsection (b); and
(2) the consideration by the Secretary of such critical comments.
* * * * * * *
Sec. 604.[42 U.S.C. 291d]
(a) Any State desiring to participate in this part may submit a State plan. Such plan must—
(1) designate a single State agency as the sole agency for the administration of the plan, or designate such agency as the sole agency for supervising the administration of the plan;
(2) contain satisfactory evidence that the State agency designated in accordance with paragraph (1) will have authority to carry out such plan in conformity with this part;
(3) provide for the designation of a State advisory council which shall include (A) representatives of nongovernmental organizations or groups, and of public agencies, concerned with the operation, construction, or utilization of hospital or other facilities for diagnosis, prevention, or treatment of illness or disease, or for provision of rehabilitation services, and representatives particularly concerned with education or training of health professions personnel, and (B) an equal number of representatives of consumers familiar with the need for the services provided by such facilities, to consult with the State agency in carrying out the plan, and provide, if such council does not include any representatives of nongovernmental organizations or groups, or State agencies, concerned with rehabilitation, for consultation with organizations, groups, and State agencies so concerned;
(4) set forth, in accordance with criteria established in regulations prescribed under section 603 and on the basis of a statewide inventory of existing facilities, a survey of need, and (except to the extent provided by or pursuant to such regulations) community, area, or regional plans—
(A) the number of general hospital beds and long-term care beds, and the number and types of hospital facilities and facilities for long-term care, needed to provide adequate facilities for inpatient care of people residing in the State, and a plan for the distribution of such beds and facilities in service areas throughout the State;
(B) the public health centers needed to provide adequate public health services for people residing in the State, and a plan for the distribution of such centers throughout the State;
(C) the outpatient facilities needed to provide adequate diagnostic or treatment services to ambulatory patients residing in the State, and a plan for distribution of such facilities throughout the State;
(D) the rehabilitation facilities needed to assure adequate rehabilitation services for disabled persons residing in the State, and a plan for distribution of such facilities throughout the State; and
(E) effective January 1, 1966, the extent to which existing facilities referred to in section 601(a) or (b) in the State are in need of modernization;
(5) set forth a construction and modernization program conforming to the provisions set forth pursuant to paragraph (4) and regulations prescribed under section 603 and providing for construction or modernization of the hospital or long-term care facilities, public health centers, outpatient facilities, and rehabilitation facilities which are needed, as determined under the provisions so set forth pursuant to paragraph (4);
(6) set forth, with respect to each of such types of medical facilities, the relative need, determined in accordance with regulations prescribed under section 603, for projects for facilities of that type, and provide for the construction or modernization, insofar as financial resources available therefor and for maintenance and operation make possible, in the order of such relative need;
(7) provide minimum standards (to be fixed in the discretion of the State) for the maintenance and operation of facilities providing inpatient care which receive aid under this part and, effective July 1, 1966, provide for enforcement of such standards with respect to projects approved by the Surgeon General under this part after June 30, 1964;
(8)[8] provide such methods of administration of the State plan, including methods relating to the establishment and maintenance of personnel standards on a merit basis (except that the Surgeon General shall exercise no authority with respect to the selection, tenure of office, or compensation of any individual employed in accordance with such methods), as are found by the Surgeon General to be necessary for the proper and efficient operation of the plan;
(9) provide for affording to every applicant for a construction or modernization project an opportunity for a hearing before the State agency;
(10) provide that the State agency will make such reports, in such form and containing such information, as the Surgeon General may from time to time reasonably require, and will keep such records and afford such access thereto as the Surgeon General may find necessary to assure the correctness and verification of such reports;
(11) provide that the Comptroller General of the United States or his duly authorized representatives shall have access for the purpose of audit and examination to the records specified in paragraph (10);
(12) provide that the State agency will from time to time, but not less often than annually, review its State plan and submit to the Surgeon General any modifications thereof which it considers necessary; and
(13) Effective[9] July 1, 1971, provide that before any project for construction or modernization of any general hospital is approved by the State agency there will be reasonable assurance of adequate provision for extended care services (as determined in accordance with regulations) to patients of such hospital when such services are medically appropriate for them, with such services being provided in facilities which (A) are structurally part of, physically connected with, or in immediate proximity to, such hospital, and (B) either (i) are under the supervision of the professional staff of such hospital or (ii) have organized medical staffs and have in effect transfer agreements with such hospital; except that the Secretary may, at the request of the State agency, waive compliance with clause (A) or (B), or both such clauses, as the case may be, in the case of any project if the State agency has determined that compliance with such clause or clauses in such case would be inadvisable.
* * * * * * *
Sec. 913. [42U.S.C. 299b-2]
(a) In General.—The Director shall—
(1) conduct a survey to collect data on a nationally representative sample of the population on the cost, use and, for fiscal year 2001 and subsequent fiscal years, quality of health care, including the types of health care services Americans use, their access to health care services, frequency of use, how much is paid for the services used, the source of those payments, the types and costs of private health insurance, access, satisfaction, and quality of care for the general population including rural residents and also for populations identified in section 299(c) of this title; and
(2) develop databases and tools that provide information to States on the quality, access, and use of health care services provided to their residents.
(b) Quality and Outcomes Information.—
(1) In general.—Beginning in fiscal year 2001, the Director shall ensure that the survey conducted under subsection (a)(1) of this section will—
(A) identify determinants of health outcomes and functional status, including the health care needs of populations identified in section 299(c) of this title, provide data to study the relationships between health care quality, outcomes, access, use, and cost, measure changes over time, and monitor the overall national impact of Federal and State policy changes on health care;
(B) provide information on the quality of care and patient outcomes for frequently occurring clinical conditions for a nationally representative sample of the population including rural residents; and
(C) provide reliable national estimates for children and persons with special health care needs through the use of supplements or periodic expansions of the survey.
In expanding the Medical Expenditure Panel Survey, as in existence on December 6, 1999, in fiscal year 2001 to collect information on the quality of care, the Director shall take into account any outcomes measurements generally collected by private sector accreditation organizations.
(2) Annual report.—Beginning in fiscal year 2003, the Secretary, acting through the Director, shall submit to Congress an annual report on national trends in the quality of health care provided to the American people.
INFORMATION SYSTEMS FOR HEALTH CARE IMPROVEMENTSec. 914.[42 U.S.C. 299b-3]
(a) In General.—In order to foster a range of innovative approaches to the management and communication of health information, the Agency shall conduct and support research, evaluations, and initiatives to advance—
(1) the use of information systems for the study of health care quality and outcomes, including the generation of both individual provider and plan-level comparative performance data;
(2) training for health care practitioners and researchers in the use of information systems;
(3) the creation of effective linkages between various sources of health information, including the development of information networks;
(4) the delivery and coordination of evidence-based health care services, including the use of real-time health care decision-support programs;
(5) the utility and comparability of health information data and medical vocabularies by addressing issues related to the content, structure, definitions and coding of such information and data in consultation with appropriate Federal, State and private entities;
(6) the use of computer-based health records in all settings for the development of personal health records for individual health assessment and maintenance, and for monitoring public health and outcomes of care within populations; and
(7) the protection of individually identifiable information in health services research and health care quality improvement.
Sec. 1301.[42 U.S.C. 300e]
(a) For purposes of this title, the term “health maintenance organization” means a public or private entity which is organized under the laws of any State and which (1) provides basic and supplemental health services to its members in the manner prescribed by subsection (b), and (2) is organized and operated in the manner prescribed by subsection (c).
* * * * * * *
(c) Each health maintenance organization shall—
* * * * * * *
(3)(A) enroll persons who are broadly representative of the various age, social, and income groups within the area it serves, except that in the case of a health maintenance organization which has a medically underserved population located (in whole or in part) in the area it serves, not more than 75 per centum of the members of that organization may be enrolled from the medically underserved population unless the area in which such population resides is also a rural area (as designated by the Secretary), and (B) carry out enrollment of members who are entitled to medical assistance under a State plan approved under title XIX of the Social Security Act in accordance with procedures approved under regulations promulgated by the Secretary;
* * * * * * *
(8) provide, in accordance with regulations of the Secretary (including safeguards concerning the confidentiality of the doctor-patient relationship), an effective procedure for developing, compiling, evaluating, and reporting to the Secretary, statistics and other information (which the Secretary shall publish and disseminate on an annual basis and which the health maintenance organization shall disclose, in a manner acceptable to the Secretary, to its members and the general public) relating to (A) the cost of its operations, (B) the patterns of utilization of its services, (C) the availability, accessibility, and acceptability of its services, (D) to the extent practical, developments in the health status of its members, and (E) such other matters as the Secretary may require.
* * * * * * *
Sec. 1302. [42 U.S.C. 300e-1]
For purposes of this title:
* * * * * * *
(7) The term “medically underserved population” means the population of an urban or rural area designated by the Secretary as an area with a shortage of personal health services or a population group designated by the Secretary as having a shortage of such services. Such a designation may be made by the Secretary only after consideration of the comments (if any) of (A) each State health planning and development agency which covers (in whole or in part) such urban or rural area or the area in which such population group resides, and (B) each health systems agency designated for a health service area which covers (in whole or in part) such urban or rural area or the area in which such population group resides.
(8)(A) The term “community rating system” means the systems, described in subparagraphs (B) and (C), of fixing rates of payments for health services. A health maintenance organization may fix its rates of payments under the system described in subparagraph (B) or (C) or under both such systems, but a health maintenance organization may use only one such system for fixing its rates of payments for any one group.
(B) A system of fixing rates of payment for health services may provide that the rates shall be fixed on a per-person or per-family basis and may authorize the rates to vary with the number of persons in a family, but, except as authorized in subparagraph (D), such rates must be equivalent for all individuals and for all families of similar composition.
(C) A system of fixing rates of payment for health services may provide that the rates shall be fixed for individuals and families by groups. Except as authorized in subparagraph (D), such rates must be equivalent for all individuals in the same group and for all families of similar composition in the same group. If a health maintenance organization is to fix rates of payment for individuals and families by groups, it shall—
(i)(I) classify all of the members of the organization into classes based on factors which the health maintenance organization determines predict the differences in the use of health services by the individuals or families in each class and which have not been disapproved by the Secretary,
(II) determine its revenue requirements for providing services to the members of each class established under subclause (I), and
(III) fix the rates of payments for the individuals and families of a group on the basis of a composite of the organization's revenue requirements determined under subclause (II) for providing services to them as members of the classes established under subclause (I), or
(ii) fix the rates of payments for the individuals and families of a group on the basis of the organization's revenue requirements for providing services to the group, except that the rates of payments for the individuals and families of a group of less than 100 persons may not be fixed at rates greater than 110 percent of the rate that would be fixed for such individuals and families under subparagraph (B) or clause (i) of this subparagraph.
The Secretary shall review the factors used by each health maintenance organization to establish classes under clause (i). If the Secretary determines that any such factor may not reasonably be used to predict the use of the health services by individuals and families, the Secretary shall disapprove such factor for such purpose.
(D) The following differentials in rates of payments may be established under the systems described in subparagraphs (B) and (C):
(i) Nominal differentials in such rates may be established to reflect differences in marketing costs and the different administrative costs of collecting payments from the following categories of members:
(I) Individual members (including their families).
(II) Small groups of members (as determined under regulations of the Secretary).
(III) Large groups of members (as determined under regulations of the Secretary).
(ii) Nominal differentials in such rates may be established to reflect the compositing of the rates of payment in a systematic manner to accommodate group purchasing practices of the various employers.
(iii) Differentials in such rates may be established for members enrolled in a health maintenance organization pursuant to a contract with a governmental authority under section 1079 or 1086 of title 10, United States Code, or under any other governmental program (other than the health benefits program authorized by chapter 89 of title 5, United States Code) or any health benefits program for employees of States, political subdivision of States, and other public entities.
* * * * * * *
Sec. 1310.[42 U.S.C. 300e-9]
(c) For purposes of this section, the term “qualified health maintenance organization” means (1) a health maintenance organization which has provided assurances satisfactory to the Secretary that it provides basic and supplemental health services to its members in the manner prescribed by section 1301(b) and that it is organized and operated in the manner prescribed by section 1301(c), and (2) an entity which proposes to becomes a health maintenance organization and which the Secretary determines will when it becomes operational provide basic and supplemental health services to its members in the manner prescribed by section 1301(b) and will be organized and operated in the manner prescribed by section 1301(c).
* * * * * * *
* * * * * * *
Sec. 1312.[42 U.S.C. 300e-11]
(a) If the Secretary determines that an entity which received a grant, contract, loan, or loan guarantee under this title as a health maintenance organization or which was included in a health benefits plan offered to employees pursuant to section 1310—
(1) fails to provide basic and supplemental services to its members,
(2) fails to provide such services in the manner prescribed by section 1301(b), or
(3) is not organized or operated in the manner prescribed by section 1301(c),
the Secretary may take the action authorized by subsection (b).
(b)(1) If the Secretary makes, with respect to any entity which provided assurances to the Secretary under section 1310(d)(1), a determination described in subsection (a), the Secretary shall notify the entity in writing of the determination. Such notice shall specify the manner in which the entity has not complied with such assurances and direct that the entity initiate (within 30 days of the date the notice is issued by the Secretary or within such longer period as the Secretary determines is reasonable) such action as may be necessary to bring (within such period as the Secretary shall prescribe) the entity into compliance with the assurances. If the entity fails to initiate corrective action within the period prescribed by the notice or fails to comply with the assurances within such period as the Secretary prescribes, then after the Secretary provides the entity a reasonable opportunity for reconsideration of his determination, including, at the entity's election, a fair hearing (A) the entity shall not be a qualified health maintenance organization for purposes of section 1310 until such date as the Secretary determines that it is in compliance with the assurances, and (B) each employer which has offered membership in the entity in compliance with section 1310, each lawfully recognized collective bargaining representative or other employee representative which represents the employees of each such employer, and the members of such entity shall be notified by the entity that the entity is not a qualified health maintenance organization for purposes of such section. The notice required by clause (B) of the preceding sentence shall contain, in readily understandable language, the reasons for the determination that the entity is not a qualified health maintenance organization. The Secretary shall publish in the Federal Register each determination referred to in this paragraph.
(2) If the Secretary makes, with respect to an entity which has received a grant, contract, loan, or loan guarantee under this title, a determination described in subsection (a), the Secretary may, in addition to any other remedies available to him, bring a civil action in the United States district court for the district in which such entity is located to enforce its compliance with the assurances it furnished respecting the provision of basic and supplemental health services or its organization or operation, as the case may be, which assurances were made in connection with its application under this title for the grant, contract, loan, or loan guarantee.
* * * * * * *
Sec. 1318.[42 U.S.C. 300e-17]
(a) Each health maintenance organization shall, in accordance with regulations of the Secretary, report to the Secretary financial information which shall include the following:
(1) Such information as the Secretary may require demonstrating that the health maintenance organization has a fiscally sound operation.
(2) A copy of the report, if any, filed with the Centers for Medicare & Medicaid Services containing the information required to be reported under section 1124 of the Social Security Act by disclosing entities and the information required to be supplied under section 1902(a)(38) of such Act.
(3) A description of transactions, as specified by the Secretary, between the health maintenance organization and a party in interest. Such transactions shall include—
(A) any sale or exchange, or leasing of any property between the health maintenance organization and a party in interest;
(B) any furnishing for consideration of goods, services (including management services), or facilities between the health maintenance organization and a party in interest, but not including salaries paid to employees for services provided in the normal course of their employment and health services provided to members by hospitals and other providers and by staff, medical group (or groups), individual practice association (or associations), or any combination thereof; and
(C) any lending of money or other extension of credit between a health maintenance organization and a party in interest.
The Secretary may require that information reported respecting a health maintenance organization which controls, is controlled by, or is under common control with, another entity be in the form of a consolidated financial statement for the organization and such entity.
(b) For the purposes of this section the term “party in interest” means:
(1) any director, officer, partner, or employee responsible for management or administration of a health maintenance organization, any person who is directly or indirectly the beneficial owner of more than 5 per centum of the equity of the organization, any person who is the beneficial owner of a mortgage, deed of trust, note, or other interest secured by, and valuing more than 5 per centum of the health maintenance organization, and, in the case of a health maintenance organization organized as a nonprofit corporation, an incorporator or member of such corporation under applicable State corporation law;
(2) any entity in which a person described in paragraph (1)—
(A) is an officer or director;
(B) is a partner (if such entity is organized as a partnership);
(C) has directly or indirectly a beneficial interest of more than 5 per centum of the equity; or
(D) has a mortgage, deed of trust, note, on[10] other interest valuing more than 5 per centum of the assets of such entity;
(3) any person directly or indirectly controlling, controlled by, or under common control with a health maintenance organization; and
(4) any spouse, child, or parent of an individual described in paragraph (1).
(c) Each health maintenance organization shall make the information reported pursuant to subsection (a) available to its enrollees upon reasonable request.
* * * * * * *
Sec. 2791.[42 U.S.C. 300gg-91(a)(1)]
(a) Group health plan.—
(1) Definition.—The term “group health plan” means an employee welfare benefit plan (as defined in section 3(1) of the Employee Retirement Income Security Act of 1974 [29 U.S.C. 1002(1)]) to the extent that the plan provides medical care (as defined in paragraph (2)) and including items and services paid for as medical care) to employees or their dependents (as defined under the terms of the plan) directly or through insurance, reimbursement, or otherwise.
(2) Medical care.—The term “medical” means amounts paid for—
(A) the diagnosis, cure, mitigation, treatment, or prevention of disease, or amounts paid for the purpose of affecting any structure or function of the body,
(B) amounts paid for transportation primarily for and essential to medical care referred to in subparagraph (A), and
(C) amounts paid for insurance covering medical care referred to in subparagraphs (A) and (B).
(b) Definitions relating to health insurance.—
* * * * * * *
* * * * * * *
(3) Health maintenance organization.—The term “health maintenance organization”—
(A) a Federally qualified health maintenance organization (as defined in section 300e(a) of this title),
(B) an organization recognized under State law as a health maintenance organization, or
(C) a similar organization regulated under State law for solvency in the same manner and to the same extent as such a health maintenance organization.
[Internal References.—SSAct §§501(b), 1101(a), 1121(a) and (c), 1122(b) and (d), 1124(a), 1128B(b), 1138(a) and (b), 1142(a) and (b), 1171, 1833(m), 1861(s), (v), and (aa), 1876(b), (e), and (i), 1883(b), 1892(a) and (b), 1903(g) and (m), 1905(l), 1927(a) and (b), 1928(d) and 2103(b) cite the Public Health Service Act. SSAct Titles V, XVIII, and XIX and §1124 heading and §1902(a) have footnotes referring to P.L. 78-410.]
* * * * * * *
[1] Sec. 208(a)(3) of P.L. 91-648 (42 U.S.C. 4728) transferred to the U.S. Civil Service Commission all functions, powers, and duties of the Secretary under any law applicable to a grant program which requires the establishment and maintenance of personnel standards on a merit basis with respect to the program.
[2] January 4, 1983 [P.L. 97-414; 96 Stat. 2049].
[3] November 4, 1992.
[4] As in original; should be “proficiency”.
[5] Probably should be “it to”.
[6] As in original; no closing punctuation.
[7] As in original. The semicolon probably should be a comma.
[8] Sec. 208(a)(3) of P.L. 91-648 (42 U.S.C. 4728) transferred to the U.S. Civil Service Commission all functions, powers, and duties of the Secretary under any law applicable to a grant program which requires the establishment and maintenance of personnel standards on a merit basis with respect to the program.
[9] As in original. Possibly should be “effective”.
[10] As in original. Possibly should be “or”.