| <<< Back to Waste Anesthetic Gases |
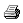 Printing Instructions
Printing Instructions
|
Anesthetic Gases: Guidelines for Workplace Exposures
|
These guidelines are not a new standard or regulation, and they create no new
legal obligations. The guidelines are advisory in nature, informational in content, and are intended to assist employers in providing a safe
and healthful workplace through effective prevention programs adapted to the needs of each place of employment. These guidelines are not
intended to address issues to patient care.
The Occupational Safety and Health Act requires employers to comply with hazard-specific safety and health
standards. In addition, employers must provide their employees with a workplace free from recognized hazards likely to
cause death or serious physical harm under Section 5(a)(1), the General Duty Clause of the Act. Employers can be cited
for violating the General Duty Clause if there is a recognized hazard and they do not take steps to prevent or abate
the hazard. However, failure to implement these guidelines is not, in itself, a violation of the General Duty Clause.
Citations can only be based on standards, regulations, and the General Duty Clause.
|
OSHA Directorate for Technical Support
Office of Science and Technical Assessment
July 20, 1999
Revised May 18, 2000 |
Table of Contents
- INTRODUCTION
- GENERAL INFORMATION
- Table 1. Inhaled Anesthetic Agents.
- HEALTH EFFECTS
- Nitrous Oxide
- Halogenated Agents
- Summary
- THE BASIC ANESTHESIA MACHINE
- Gas Flow in the Anesthesia Machine and Breathing System
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
- Sources of Leaks Within the Anesthesia Machine and Breathing System
- Checking Anesthesia Machines
- GENERAL WORKPLACE CONTROLS
- Engineering Controls
- Work Practices
- Administrative Controls
- Personal Protective Equipment
- LOCATION-SPECIFIC WORKPLACE CONTROLS
- Hospital Operating Rooms
Figure 6
- Postanesthesia Care in Hospitals and Stand-Alone Facilities
- Dental Operatory
Figure 7
- Veterinary Clinics and Hospitals
Figure 8
- CLEAN-UP AND DISPOSAL OF LIQUID ANESTHETIC AGENT SPILLS
- AIR MONITORING
- Time-Integrated Sampling
- Real-Time Sampling
- Additional Sampling Guidelines
- MEDICAL SURVEILLANCE
- HAZARD COMMUNICATION
- REFERENCES
APPENDIX 1. Glossary.
APPENDIX 2. Food and Drug Administration (FDA) Anesthesia Apparatus Checkout Recommendations, 1993.
APPENDIX 3. Scavenging System, Interface Component.
A. INTRODUCTION
This document provides general information and guidance about anesthetic gases and
workplace exposures. Workplace exposures to anesthetic gases occur in hospital-based
and stand-alone operating rooms, recovery rooms, dental operatories, and veterinary
facilities. Engineering, work practice, and administrative controls that help reduce these
exposures in all anesthetizing locations, are identified and discussed. Sources of leaks in
anesthesia equipment systems, components, and accessories are identified and appropriate
methods are described that limit excessive leaks.
Inhaled anesthetic agents include two different classes of chemicals: nitrous oxide and
halogenated agents. Halogenated agents currently in use include halothane
(Fluothane®), enflurane (Ethrane®),
isoflurane (Forane®), desflurane (Suprane®),
and sevoflurane (Ultane®). Methoxyflurane (Penthrane®),
once in general use, is now only infrequently used primarily in veterinary procedures. At
present, the Occupational Safety and Health Administration (OSHA) has no permissible
exposure limits regulating these agents.
In 1977, the National Institute for Occupational Safety and Health (NIOSH) issued
recommended exposure limits (RELs) for both nitrous oxide and halogenated agents. The
NIOSH REL for nitrous oxide, when nitrous oxide is used as the sole inhaled anesthetic
agent, is 25 parts per million (ppm) measured as a time-weighted average
(TWA) during the period of anesthetic administration (NIOSH 1977).
That recommendation remains in effect. [The American Dental Association points out
that Dr. D. Bruce, who conducted the 1974 study upon which the REL was based, said in
letters to the editor published in Anesthesia Analgesia (1983) and
Anesthesiology (1991) that he no longer believes his conclusions to be
valid and that the"NIOSH standards should be revised."]
NIOSH also recommended that no worker should be exposed at ceiling concentrations
greater than 2 ppm of any halogenated anesthetic agent over a sampling period not to
exceed one hour. In 1989, the American Conference of Governmental Industrial Hygienists
(ACGIH) assigned a threshold limit value-time-weighted average
(TLV-TWA) for nitrous oxide of 50 ppm for a normal 8-hour
workday. ACGIH TLV-TWAs also exist for halothane and enflurane, and are
50 ppm and 75 ppm, respectively. No NIOSH REL's exist for the three most currently
used anesthetics (isoflurane, desflurane, and sevoflurane).
It is the intention of this document to provide helpful information on protecting the health and safety of
anesthesiologists, nurse anesthetists and operating and recovery room personnel working
around the administration of anesthetic gases. Sections that discuss general workplace
controls, location-specific workplace controls, monitoring, a suggested
medical surveillance program, hazard communication and training, and the management of
spills and leaks and their appropriate disposal are designed to reduce workers’ exposure
to, and related health risks from, inadequately controlled waste anesthetic gases.
These guidelines are not a new standard or regulation. They are advisory in
nature, informational in content, and intended for use by employers in providing a safe
and healthful workplace through effective prevention programs adapted to the needs
and resources of each place of employment. In addition, it is recognized that the
patient’s welfare, safety, and rights of privacy are paramount. The recommendations presented
in this document should in no way preclude proper patient care and safety, particularly if
patient needs arise that require deviation from these guidelines. The guidelines are not
meant to compromise safe anesthetic practices.
B. GENERAL INFORMATION
Surgical inhalation anesthesia was first used in the United States when diethyl ether
was administered to a patient in 1842. Since then, many chemical compounds have been used
to anesthetize patients to keep them free from pain during surgical procedures. Many
anesthetic agents such as diethyl ether, divinyl ether, cyclopropane, and ethylene, were
effective in their intended use but posed a fire and explosion risk in the presence of a
sufficient oxygen supply and an ignition source such as a spark from static electricity or
electrical equipment.
In the 1950s, developments in chlorofluorocarbon chemistry produced halogenated,
nonflammable, volatile agents that replaced the explosive agents. More than 20 years ago
the Joint Commission on Accreditation of Hospitals (JCAH) in its"Accreditation Manual for
Hospitals" prohibited the use of flammable anesthetic agents in all anesthetizing
locations. Table 1 lists inhaled anesthetic agents that have been used in the past and
those that are currently in use.
Table 1. Inhaled Anesthetic Agents
| Generic or chemical name |
Commercial name |
Year of introduction |
Currently in use? |
| Diethyl ether |
Ether |
1842 |
No |
| Nitrous oxide |
Nitrous oxide |
1844 |
Yes |
| Chloroform |
Chloroform |
1847 |
No |
| Cyclopropane |
Cyclopropane |
1933 |
No |
| Trichloroethylene |
Trilene® |
1934 |
No |
| Fluroxene |
Fluoromar® |
1954 |
No |
| Halothane |
Fluothane® |
1956 |
Yes |
| Methoxyflurane |
Penthrane® |
1960 |
Infrequently |
| Enflurane |
Ethrane® |
1974 |
Yes |
| Isoflurane |
Forane® |
1980 |
Yes |
| Desflurane |
Suprane® |
1992 |
Yes |
| Sevoflurane |
Ultane® |
1995 |
Yes |
It is estimated that more than 200,000 health care professionals --including
anesthesiologists, nurse anesthetists, surgical and obstetric nurses, operating room (OR)
technicians, nurses aides, surgeons, anesthesia technicians, postanesthesia care nurses,
dentists, dental assistants, dental hygienists, veterinarians and their assistants,
emergency room staff, and radiology department personnel --are potentially exposed to waste
anesthetic gases and are at risk of occupational illness. Over the years there have been
significant improvements in the control of anesthetic gas pollution in
health-care facilities. These have been accomplished through the use and
improved design of scavenging systems, installation of more effective general ventilation
systems, and increased attention to equipment maintenance and leak detection as well as to
careful anesthetic practice. However, occupational exposure to waste gases still
occurs.
Exposure measurements taken in ORs during the clinical administration of inhaled
anesthetics indicate that waste gases can escape into the room air from various components
of the anesthesia delivery system. Potential leak sources include tank valves,
high- and low-pressure machine connections; connections in the
breathing circuit, defects in rubber and plastic tubing, hoses, reservoir bags, and
ventilator bellows, and the Y-connector. In addition, selected anesthesia
techniques and improper practices such as leaving gas flow control valves open and
vaporizers on after use, spillage of liquid inhaled anesthetics, and poorly fitting face
masks or improperly inflated tracheal tube and laryngeal mask airway cuffs also can
contribute to the escape of waste anesthetic gases into the OR atmosphere.
Studies of the effects of these agents in the health-care setting have been
made more difficult due to high job turnover of affected employees. Publications report a
wide range of exposure levels in hospital, medical, dental, and veterinary facilities
(Askrog and Petersen 1970;
American Society of Anesthesiologists 1974;
Sweeney et al. 1985;
Jastak 1989;
Burkhart and Stobbe 1990;
Henry and Jerrell 1990;
Rowland et al. 1992;
NIOSH 1977,
1994).
Unlike the situation in the OR, health-care workers in the recovery room
(also known as the postanesthesia care unit or PACU) encounter occupational exposure to
waste anesthetic gases from the patients instead of the anesthesia delivery system. While
in the OR, patients anesthetized with inhaled anesthetic agents take-up
varying quantities of these agents depending on the specific agent and its solubility, the
duration of anesthesia, and the physiological make-up of the patient. In the
PACU, these gases are eliminated by the patient’s respiratory system into the ambient
environment. In contrast to the OR, the ambient air in the PACU may contain multiple
anesthetic gases, which include but are not limited to nitrous oxide, halothane, enflurane,
isoflurane, desflurane, and sevoflurane.
Because PACU nurses must monitor vital functions in close physical proximity to the
patient, they can be exposed to measurable concentrations of waste anesthetic gases. While
random room samples may indicate relatively low levels of waste gases, the breathing zone
of the nurses may contain higher levels.Consequently, air samples obtained within
the breathing zone of a nurse providing bedside care are most likely to represent the gas
concentrations actually inhaled.
In general, the detection of halogenated anesthetic agents by their odor would indicate
the existence of very high levels, as these agents do not have a strong odor at low
concentrations. For example, detection of high levels of halothane may be difficult for
PACU nurses because one study (Hallen et al. 1970) found that fewer than
50% of the population can detect the presence of halothane until concentrations are 125
times the NIOSH REL.
C. HEALTH EFFECTS
In anesthetizing locations and PACUs where exposure to waste gases is known to occur, it
is important for health-care workers and their employers to understand the
potential risks of excess exposure to waste anesthetic gases and to implement the
appropriate controls to minimize these risks. During the past 25 years multiple studies have
attempted to elucidate the risk of exposure to anesthetic agents. Animal and human studies
have assessed hematopoietic, central nervous system, and behavioral effects and the effects
of anesthetic agents on fertility, carcinogenicity, teratogenicity, and reproduction.
Epidemiological studies have generally focused on OR and dental workers, the two occupational
groups most frequently exposed to anesthetics. The following discussion highlights these findings.
- Nitrous Oxide
While mutagenicity testing of nitrous oxide (N2O) has
demonstrated negative results (Baden 1980), reproductive and teratogenic
studies in several animal species have raised concern about the possible effects of nitrous
oxide exposure in humans. In general, studies demonstrate reproductive and developmental
abnormalities in animals exposed to high concentrations ofN2O. In
one study by Viera et al. (1980), spontaneous abortion was observed in rats
at 1000 ppm or more. According to NIOSH (1994), similar concentrations of
1000 ppm have been found in operating rooms and in dental operatories not equipped with
scavenging systems.
Smith, Gaub, and Moya (1965) reported fetal resorption in rats exposed
to nitrous oxide at high doses. Fink, Shepard, and Blandau (1967)
administered 45% to 50% nitrous oxide and 21% to 25% oxygen to pregnant rats for 2, 4, and 6
days starting at day 8 of gestation. Surviving fetuses from these rats demonstrated rib and
vertebral defects. Corbett and colleagues (1973) also reported an increase
in fetal deaths and a smaller number of offspring in rats exposed to levels ranging from
1,000 to 15,000 ppm of nitrous oxide.
There are also studies involving human subjects. A recent retrospective study (Rowland et al. 1992)
reported that female dental assistants exposed to
unscavenged N2O for 5 or more hours per week had a significantly
increased risk of reduced fertility compared with non-exposed female dental
assistants. The exposed assistants had a 59% decrease in probability of conception for any
given menstrual cycle compared with the non-exposed assistants. For dental
assistants who used scavenging systems during N2O administration,
the probability of conception was not significantly different from that of the
non-exposed assistants. The Rowland study authors suggest that"exposure to
high levels of unscavenged N2O can impair fertility and scavenging
equipment is important in protecting the reproductive health of women who work with the
gas." The study revealed that the mean time to conception among the women who worked with
scavengedN2O was similar to that among the non-exposed
women, but it was much longer among the women who worked with unscavenged N2O
for 5 or more hours a week.
Rowland and colleagues (1995) examined the relationship between
occupational exposure to N2O and spontaneous abortion in female
dental assistants. Duration of exposure was a surrogate for exposure data. Nitrous oxide
exposure was divided into two separate variables: scavenged hours (hours of exposure per
week in the presence of scavenging equipment) and unscavenged hours of exposure per week.
Women who worked with N2O at least 3 hours per week in offices
not using scavenging equipment had an increased risk of spontaneous abortion (relative risk
= 2.6, 95% confidence interval [CI] = 1.3-5.0) adjusted for age, smoking, and
number of amalgams prepared per week. This finding was not observed among workers in
offices where scavenging equipment was in use. The authors concluded,"Scavenging equipment
can make large differences in exposure levels at moderate cost and appears to be important
in protecting the reproductive health of women who work with nitrous oxide."
Several summaries of the epidemiologic studies of exposure to
N2O and reviews of the topic generally including animal and
retrospective studies (Purdham 1986; Kestenberg 1988;
and NIOSH 1994) have been published. They report a consistent excess of
spontaneous abortion in exposed women. Other summaries of the epidemiologic studies do not
establish a cause-effect relationship (Buring et al. 1985; Tannenbaum and
Goldberg 1985). Evidence for congenital abnormalities is less strongly associated with
exposure.
- Halogenated Agents
Halogenated agents are used with and without N2O and have been
linked to reproductive problems in women and developmental defects in their offspring. As
early as 1967 there were reports from the Soviet Union, Denmark, and the United States
(Vaisman 1967; Askrog and Petersen 1970;
Cohen, Bellville, and Brown 1971) that exposure to anesthetic agents
including halothane may cause adverse pregnancy outcomes in health-care
personnel. Several animal studies in rats, mice and hamsters showed embryolethal and
teratogenic effects and supported the findings in humans (Basford and Fink 1968;
Wharton 1979), although often at quite high concentrations (3000-6000
ppm). One (Popova et al. 1979) reported fetal resorptions in rats at 9 ppm.
A number of human epidemiologic studies have been performed since the early 1970s to
assess the potential harm to reproductive health that exposure to anesthetics might cause.
Generally, these were mailed questionnaire surveys completed by persons (usually
anesthesiologists and nurses) identified through registries. As such, the studies were
retrospective and inquired about previous reproductive outcomes for which validation was
not available. In addition, no exposure data were available and many of the early studies
predated the use of scavenging systems. Studies documenting a statistically significant
excess of spontaneous abortions in exposed female anesthesiologists include those of Cohen
and colleagues 1971, Knill-Jones and colleagues 1972, ASA 1974, and Pharoah
and colleagues 1977. Studies also documented increases in spontaneous abortion among
nonphysician female OR personnel (Cohen et al. 1971;
Rosenberg and Kirves 1973;
ASA 1974; Knill-Jones et al. 1975;
and Tomlin 1979). Also of interest, one study documented
increased incidence rates of spontaneous abortion among wives of exposed males
(ASA 1974). In some exposed populations, studies failed to show that
exposure to anesthetic agents caused increased risk of spontaneous abortion
(Rosenberg and Vanttinnen 1978; Axelsson and Rylander 1982;
Tannenbaum and Goldberg 1985; Buring et al. 1985).
The evidence for an association between anesthetic exposure and congenital anomalies is
less consistent. Only a few studies in some subpopulations of exposed workers found a
positive association (Corbett et al. 1974; ASA 1974;
Pharoah et al. 1977). Other studies reported no association with
congenital anomalies (Axelsson and Rylander 1982;
Lauwerys et. al. 1981; Cohen et. al. 1980;
Rosenberg and Vanttinnen 1978).
The retrospective study by Cohen and colleagues (1980) reported that
female dental chairside assistants who had experienced heavy exposure (defined as more than
eight hours per week) to waste anesthetic gases reported a significant increase in the rate
of spontaneous abortions (19.1 per 100 pregnancies) compared with the rate in the
non-exposed pregnant control (8.1 per 100). For the wives of dentists who had
also experienced heavy exposure, a significant increase in the rate of spontaneous
abortions (10.2 per 100) was also reported compared with the rate in the wives of dentists
not exposed (6.7 per 100). The non-exposed group was restricted to those who
did not report anesthetic exposure in any of the years before conception and including the
year of conception.
Another study of reproductive outcomes associated with exposure to anesthetic gases (also a questionnaire
survey, conducted between 1981 and 1985) documented both a statistically significantly increased odds ratio for
spontaneous abortion in exposed females (odds ratio 1.98; CI = 1.53-2.56) and spouses of exposed male
workers (odds ratio 2.30; CI = 1.68-3.13), and for congenital abnormality in offspring of exposed females \
(odds ratio 2.24; CI = 1.69-2.97) and offspring of spouses of exposed male workers
(odds ratio 1.46; CI = 1.04-2.05) (Guirgis et al. 1990).Duration of exposure
as estimated by a hygiene investigation was used as an exposure surrogate. These findings
of a positive association were surprising because scavenging systems were thought to have
been more likely in use during the study period compared to many of the previously cited
papers, almost a decade older.
In the mid 1970's, human studies testing the cognitive and the motor skills of male
subjects/volunteers, showed that exposure to concentrations of anesthetic gas mixtures
commonly found in the unscavenged operating room, resulted in decreased ability to perform
complex tasks (Bruce et al. 1974, 1975, later invalidated
by the author, 1983, 1991). These volunteers exhibited
decrements in performance following exposures at: 500 ppm N2O in
air; 500 ppm N2O plus 15 ppm halothane in air; and 500 ppm
N2O plus 15 ppm enflurane in air. However, studies that attempted
to replicate the results of the human performance studies that showed decrements failed to
confirm these findings (Smith and Shirley 1978).
Potential harmful effects due to desflurane exposure have been addressed in a few recent
studies, including those of Holmes and colleagues (1990), an animal study;
and Weiskopf and colleagues (1992), a study conducted with human volunteers.
However, desflurane’s potential as a hazard to health-care personnel has not
been thoroughly evaluated. Sevoflurane (Ultane®), the newest
anesthetic agent in clinical practice, has also not been thoroughly evaluated. The levels of
risk for isoflurane, desflurane, and sevoflurane have not been established. Since there are
limited data, occupational exposure limits for these agents have not been determined.
Therefore, until more information is available, it is prudent to attempt to minimize
occupational exposure to these as with all anesthetic agents.
Unlike N2O, there is evidence that halothane is mutagenic in
certain in vitro test systems (Garro and Phillips 1978) and that halothane
is metabolized to reactive intermediates that covalently bind to cellular macromolecules,
suggesting potential mechanisms of toxicity (Gandolfi et al. 1980).
- Summary
Despite questions about design issues or selection bias in some studies, the weight of
the evidence regarding potential health risks from exposure to anesthetic agents in
unscavenged environments suggests that clinicians need to be concerned. Moreover, there is
biological plausibility that adds to the concern that high levels of unscavenged waste
anesthetic gases present a potential for adverse neurological effects or reproductive risk
to exposed workers or developmental anomalies in their offspring (Cohen et al. 1980;
Rowland 1992).
While the use of prospective studies and carefully designed research protocols is
encouraged to elucidate areas of controversy, a responsible approach to worker health and
safety dictates that any exposure to waste and trace gases should be kept to the lowest
practical level.
D. THE BASIC ANESTHESIA MACHINE
An anesthesia machine is an assembly of various components and devices that include
medical gas cylinders in machine hanger yokes, pressure regulating and measuring devices,
valves, flow controllers, flow meters, vaporizers, CO2 absorber
canisters, and breathing circuit assembly. The basic two-gas anesthesia
machine has more than 700 individual components.
The anesthesia machine is a basic tool of the anesthesiologist/anesthetist and serves as
the primary work station. It allows the anesthesia provider to select and mix measured
flows of gases, to vaporize controlled amounts of liquid anesthetic agents, and thereby
to administer safely controlled concentrations of oxygen and anesthetic gases and vapors
to the patient via a breathing circuit. The anesthesia machine also provides a working
surface for placement of drugs and devices for immediate access and drawers for storage of
small equipment, drugs, supplies, and equipment instruction manuals. Finally, the machine
serves as a frame and source of pneumatic and electric power for various accessories such
as a ventilator, and monitors that observe or record vital patient functions or that are
critical to the safe administration of anesthesia.
- Gas Flow in the Anesthesia Machine and Breathing System
The internal piping of a basic two-gas anesthesia machine is shown in
Figure 1. The machine has many connections and potential sites for
leaks. Both oxygen and N2O may be supplied from two sources
(Figure 2): a pipeline supply source (central piping system from bulk storage)
and a compressed gas cylinder supply source. In hospitals, the pipeline supply source is the primary gas source for the
anesthesia machine. Pipeline supplied gases are delivered through wall outlets at a pressure of 50-55 psig
through diameter indexed safety system (DISS) fittings or through quick-connect couplings that are
gas-specific within each manufacturer's patented system.
Because pipeline systems can fail and because the machines may be used in locations
where piped gases are not available, anesthesia machines are fitted with reserve cylinders
of oxygen and N2O. The oxygen cylinder source is regulated from
approximately 2,200 psig in the tanks to approximately 45 psig in the machine
high-pressure system, and the N2O cylinder source is
regulated from 745 psig in the tanks to approximately 45 psig in the machine
high-pressure system.
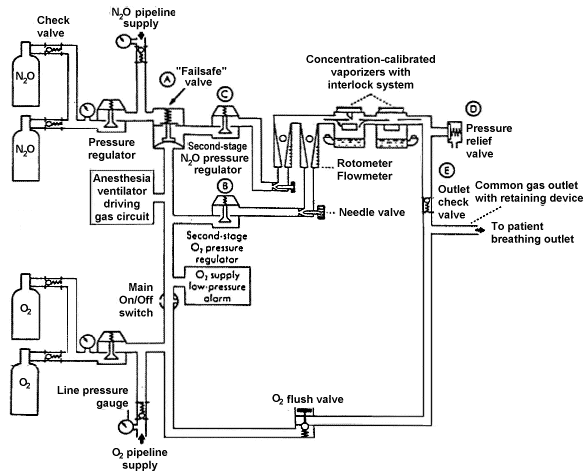
Figure 1
The flow arrangement of a basic two-gas
anesthesia machine. A, The fail-safe valve in Ohmeda machines is termed
a pressure sensor shut-off valve; in Dräger machines it is the oxygen
failure protection device (OFPD). B, Second-stage oxygen pressure
regulator is used in Ohmeda (but not Dräger Narkomed) machines. C,
Second-stage nitrous oxide pressure regulator is used in Ohmeda Modulus
machines having the Link 25 Proportion Limiting System; not used in Dräger machines.
D, Pressure relief valve used in certain Ohmeda machines; not used in Dräger
machines. E, Outlet check valve used in Ohmeda machines except Modulus II Plus and
Modulus CD models; not used in Dräger machines. The oxygen take-off for
the anesthesia ventilator driving gas circuit is downstream of the main on/off
switch in Dräger machines, as shown here. In Ohmeda machines, the
take-off is upstream of the main on/off switch.
(Adapted from
Check-out: a guide for preoperative inspection of an anesthesia machine, ASA,
1987. Reproduced by permission of the American Society of Anesthesiologists, 520 N.
Northwest Highway, Park Ridge, Ill.)
|
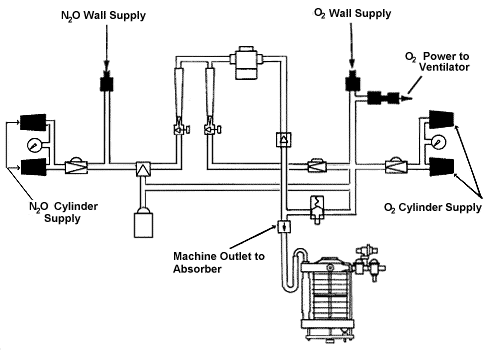
Figure 2
The supply of nitrous oxide and oxygen may come from two
sources: the wall (pipeline) supply and the reserve cylinder supply. (Reproduced by
permission of Datex·Ohmeda, Madison, Wisconsin).
|
Compressed gas cylinders of oxygen, N2O, and other medical
gases are attached to the anesthesia machine through the hanger yoke assembly. Each hanger
yoke is equipped with the pin index safety system, a safeguard introduced to eliminate
cylinder interchanging and the possibility of accidentally placing the incorrect gas tank
in a yoke designed for another gas tank.
Figure 3 shows the oxygen pathway through the flowmeter, the agent vaporizer, and the
machine piping, and into the breathing circuit. Oxygen from the wall outlet or cylinder
pressurizes the anesthesia delivery system. Compressed oxygen provides the needed energy
for a pneumatically powered ventilator, if used, and it supplies the oxygen flush valve
used to supplement oxygen flow to the breathing circuit. Oxygen also"powers" an
in-line pressure-sensor shutoff valve ("fail-safe"
valve) for other gases to prevent their administration if the O2
supply pressure in the O2 high pressure system falls below a
threshold value.
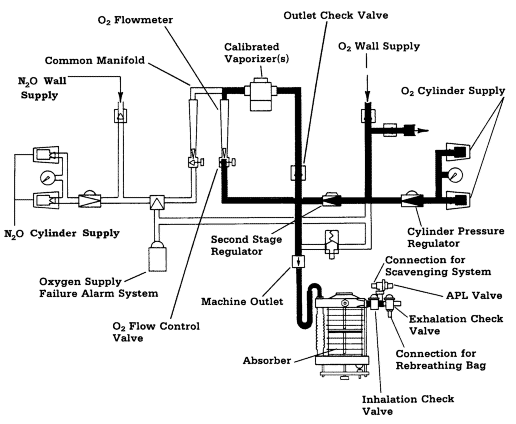
Figure 3
Oxygen and N2O flow from their
supply sources via their flow control valves, flowmeters and common manifold to the
concentration-calibrated vaporizer and then via the machine common gas
outlet to the breathing system. The high pressure system of the anesthesia machine
comprises those components from the compressed gas supply source to the gas
(O2 and N2O) flow control valves.
The low pressure system of the anesthesia machine comprises those components downstream
of the gas flow control valves.
(Reproduced by permission of Datex·Ohmeda, Madison,
Wisconsin).
|
Once the flows of oxygen, N2O, and other medical gases (if
used) are turned on at their flow control valves, the gas mixture flows into the common
manifold and through a concentration-calibrated agent-specific
vaporizer where a potent inhaled volatile anesthetic agent is added. The mixture of gases
and vaporized anesthetic agent then exits the anesthesia machine low pressure system
through the common gas outlet and flows to the breathing system.
The circle system shown in Figure 4 is the breathing system most commonly used in
operating rooms (ORs). It is so named because its components are arranged in a circular
manner. The essential components of a circle breathing system (Figure 5)
include a site for inflow of fresh gas (common [fresh] gas inlet), a carbon dioxide
absorber canister (containing soda lime or barium hydroxide lime) where exhaled carbon
dioxide is absorbed; a reservoir bag; inspiratory and
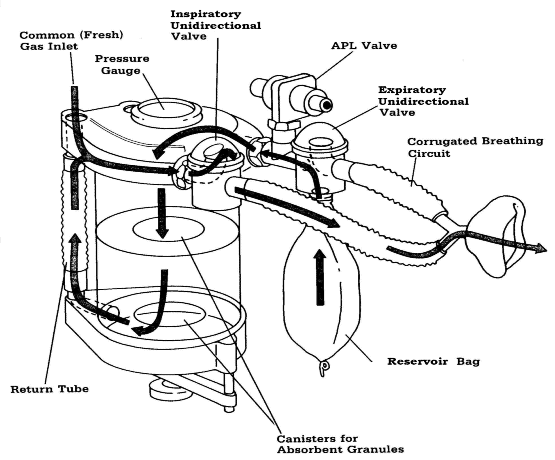
Figure 4
Basic circle breathing system.
(Reproduced by permission of Datex·Ohmeda, Madison, Wisconsin).
|
expiratory unidirectional valves; flexible corrugated breathing tubing; an adjustable
pressure-limiting (APL) or "pop-off" valve for venting excess gas;
and a"Y" piece that connects to a face mask, tracheal tube, laryngeal mask airway
(LMA) or other airway management device.
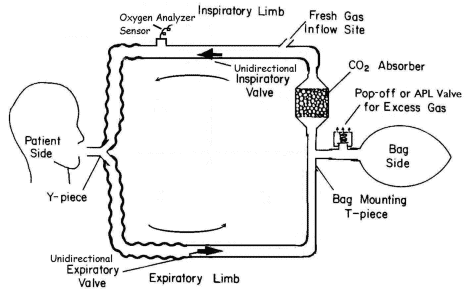
Figure 5
Essential components of a circle breathing system.
(Adapted
from Principles of Anesthesiology: general and regional anesthesia, Collins, Vincent J.,
M.D., Executive Editor: Cann, Carroll C., 1993. Reproduced by permission of Lippincott
Williams and Wilkins, Malvern, Pennsylvania).
|
Once inside the breathing system, the mixture of gases and vapors flows to the breathing
system’s inspiratory unidirectional valve, then on toward the patient. Exhaled gases pass
through the expiratory unidirectional valve and enter the reservoir bag. When the bag is
full, excess gas flows through the APL (or pop-off) valve and into the
scavenging system that removes the waste gases. On the next inspiration, gas from the
reservoir bag passes through the carbon dioxide absorber prior to joining the fresh gas
from the machine on its way to the patient. The general use of fresh gas flow rates into
anesthetic systems in excess of those required to compensate for uptake, metabolism,
leaks, or removal of exhaled carbon dioxide results in variable volumes of anesthetic
gases and vapors exiting the breathing system through the APL valve.
When an anesthesia ventilator is used, the ventilator bellows functionally replaces the
circle system reservoir bag and becomes a part of the breathing circuit. The APL valve in
the breathing circuit is either closed or excluded from the circuit using a manual
("bag")/automatic (ventilator) circuit selector switch. The ventilator incorporates a
pressure-relief valve, that permits release of excess anesthetic gases from
the circuit at end-exhalation. These gases should also be scavenged.
- Sources of Leaks Within the Anesthesia Machine and Breathing System
No anesthesia machine system is totally leak-free (Emergency Care Research
Institute 1991). Leakage may originate from both the high-pressure and
low-pressure systems of the anesthesia or analgesia machine.
The high-pressure system consists of all piping and parts of the machine that receive
gas at cylinder or pipeline supply pressure. It extends from the high-pressure
gas supply (i.e., wall supply or gas cylinder) to the flow control valves. Leaks may occur
from the high-pressure connections where the supply hose connects to the wall
outlet or gas cylinder and where it connects to the machine inlet. Therefore,
gas-supply hoses should be positioned to prevent strain on the fittings (ASTM
Standard F1161-88; Dorsch and Dorsch 1994) and constructed from supply-hose
materials designed for high-pressure gas flow and minimal kinking (Bowie and
Huffman 1985). High-pressure leakage may also occur within the anesthesia
machine itself. Other potential sources of leaks include quick-connect
fittings, cylinder valves, absent or worn gaskets, missing or worn yoke plugs in a dual
yoke assembly, and worn hoses.
The low-pressure system of the anesthesia machine (in which the pressure is slightly
above atmospheric) consists of components downstream of the flow-control
valves. It therefore includes the flow meter tubes, vaporizers, common gas outlet and
breathing circuit, (i.e., from the common gas outlet to the patient).
Low-pressure system leaks may occur from the connections and components
anywhere between the anesthesia gas flow control valves and the airway. This leakage may
occur from loose-fitting connections, defective and worn seals and gaskets,
worn or defective breathing bags, hoses, and tubing, loosely assembled or deformed slip
joints and threaded connections, and the moisture drainage port of the
CO2 absorber, which may be in the"open" position.
Low-pressure system leaks also may occur at the gas analysis sensor (i.e., circuit
oxygen analyzer) and gas sampling site(s), face mask, the tracheal tube (especially in
pediatric patients where a leak is required around the uncuffed tracheal tube), laryngeal
mask airway (over the larynx), and connection points for accessory devices such as a
humidifier, temperature probe, or positive end-expiratory pressure (PEEP)
valve. Inappropriate installation of a calibrated vaporizer(s) or misalignment of a
vaporizer on its manifold (ECRI 1991) can also contribute to anesthetic
gas leakage.
Minute absorbent particles that may have been spilled on the rubber seal around the
absorber canister(s) may also prevent a gas-tight seal when the
canister(s) in the carbon dioxide absorber is (are) reassembled (Eichhorn 1993). The
exhaust from a sidestream sampling respiratory gas analyzer and/or capnograph should also
be connected to the waste gas scavenging system because the analyzed gas sample may contain
N2O or halogenated vapors.
- Checking Anesthesia Machines
Prior to induction of anesthesia, the anesthesia machine and its components/accessories
should be made ready for use. All parts of the machine should be in good working order with
all accessory equipment and necessary supplies on hand. The waste gas disposal system
should be connected, hoses visually inspected for obstructions or kinks, and proper
operation determined. Similarly, the anesthesia breathing system should be tested to verify
that it can maintain positive pressure. Leaks should be identified and corrected before the
system is used (Bowie and Huffman 1985;
Food and Drug Administration 1993;
Dorsch and Dorsch 1994). The ability of the
anesthesia system to maintain constant pressure is tested not only for the safety of the
patient dependent on a generated positive pressure ventilation but also to test for leaks
and escape of anesthetic gases, which may expose health-care personnel to
waste anesthetic gases.
Several check-out procedures exist. The 1993 Food and Drug Administration
(FDA) Anesthesia Apparatus Checkout Recommendations Document which is shown in
Appendix 2, is based on guidelines developed by the FDA, as advised
by anesthesiologists and manufacturers. This checkout serves only as a generic guideline
because the designs of different machines and monitors vary considerably. The guideline
encourages users to modify the recommendations to accommodate differences in equipment
design, modifications, and variations in local clinical practice. The user must refer to
the machine manufacturer's operator’s manual for the manufacturer’s specific procedures or
precautions.
E. GENERAL WORKPLACE CONTROLS
Occupational exposures can be controlled by the application of a number of
well-known principles including engineering and work practice controls,
administrative controls, personal protective equipment, and monitoring. These principles
may be applied at or near the hazard source, to the general workplace environment, or at
the point of occupational exposure to individuals. Controls applied at the source of the
hazard, including engineering and work practice controls, are generally the preferred and
most effective means of control. In anesthetizing locations and PACUs, where employees are
at risk of exposure to waste anesthetic gases, exposure may be controlled by some or all of
the following: (1) effective anesthetic gas scavenging systems that remove excess
anesthetic gas at the point of origin; (2) effective general or dilution ventilation; (3)
good work practices on the part of the health-care workers, including the
proper use of controls; (4) proper maintenance of equipment to prevent leaks; and (5)
periodic personnel exposure and environmental monitoring to determine the effectiveness of
the overall waste anesthetic gas control program.
The following is a general discussion of engineering controls, work practices,
administrative controls, and personal protective equipment that can reduce worker exposure
to waste anesthetic gases. However, not every control listed in this section may be
feasible in all settings. Additional location-specific controls and
appropriate exceptions are addressed in section F.
- Engineering Controls
The collection and disposal of waste anesthetic gases in operating rooms and
non-operating room settings is essential for reducing occupational
exposures. Engineering controls such as an appropriate anesthetic gas scavenging
system are the first line of defense and the preferred method of control to protect
employees from exposure to anesthetic gases. An effective anesthetic gas scavenging
system traps waste gases at the site of overflow from the breathing circuit and
disposes of these gases to the outside atmosphere. The heating, ventilating, and air
conditioning (HVAC) system also contributes to the dilution and removal of waste
gases not collected by the scavenging system or from other sources such as leaks in
the anesthetic apparatus or improper work practices.
The exhalation of residual gases by patients in the PACU may result in significant
levels of waste anesthetic gases when appropriate work practices are not used at the
conclusion of the anesthetic or inadequate ventilation exists in the PACU. A
nonrecirculating ventilation system can reduce waste gas levels in this area. Waste
gas emissions to the outside atmosphere must meet local, state, and Environmental
Protection Agency (EPA) regulatory requirements.
A scavenging system consists of five basic components (ASTM, F 1343 - 91):
- A gas collection assembly such as a collection manifold or a distensible
bag (i.e., Jackson-Rees pediatric circuit), which captures excess
anesthetic gases at the site of emission, and delivers it to the transfer tubing.
- Transfer tubing, which conveys the excess anesthetic gases to the interface.
- The interface, which provides positive (and sometimes negative) pressure
relief and may provide reservoir capacity. It is designed to protect the patient's
lungs from excessive positive or negative scavenging system pressure.
- Gas disposal assembly tubing, which conducts the excess anesthetic
gases from the interface to the gas disposal assembly.
- The gas disposal assembly, which conveys the excess gases to a point
where they can be discharged safely into the atmosphere. Several methods in use
include a nonrecirculating or recirculating ventilation system, a central vacuum
system, a dedicated (single-purpose) waste gas exhaust system, a passive
duct system, and an adsorber.
In general, a machine-specific interface must be integrated with a
facility’s system for gas removal. The interface permits excess gas to be collected in a
reservoir (bag or canister) and limits the pressure within the bag or canister. A
facility’s gas disposal system receives waste anesthetic gases from the interface and
should vent the waste gases outside the building and away from any return air ducts or
open windows, thus preventing the return of the waste gases back into the facility.
(Refer to Appendix 3 for a more detailed description of how the
scavenging interface works.)
Removal of excess anesthetic gases from the anesthesia circuit can be accomplished by
either active or passive scavenging. When a vacuum or source of negative
pressure is connected to the scavenging interface, the system is described as an active
system. When a vacuum or negative pressure is not used, the system is described as a
passive system. With an active system there will be a negative pressure in the gas
disposal tubing. With a passive system, this pressure will be increased above atmospheric
(positive) by the patient exhaling passively, or manual compression of the breathing
system reservoir bag.
Use of a central vacuum system is an example of an active system: The waste anesthetic
gases are moved along by negative pressure. Venting waste anesthetic gas via the exhaust
grille or exhaust duct of a nonrecirculating ventilation system is an example of a passive
system: The anesthetic gas is initially moved along by the positive pressure from the
breathing circuit until it reaches the gas disposal assembly.
Active Systems
Excess anesthetic gases may be removed by a central vacuum system (servicing the ORs in
general) or an exhaust system dedicated to the disposal of excess gases. When the waste
anesthetic gas scavenging system is connected to the central vacuum system (which is
shared by other users, e.g., surgical suction), exposure levels may be effectively
controlled. The central vacuum system must be specifically designed to handle the large
volumes of continuous suction from OR scavenging units. If a central vacuum system is
used, a separate, dedicated gas disposal assembly tubing should be used for the scavenging
system, distinct from the tubing used for patient suctioning (used for oral and nasal
gastric sources as well as surgical suctioning).
Similarly, when a dedicated exhaust system (low velocity) is used, excess gases can
also be collected from one or more ORs and discharged to the outdoors. The exhaust fan
must provide sufficient negative pressure and air flow so that
cross-contamination does not occur in the other ORs connected to this system.
Active systems are thought to be more effective than passive systems at reducing excess
waste anesthetic gas concentrations because leaks in the scavenging system do not result
in an outward loss of gas.
Passive Systems
HVAC systems used in health-care facilities are of two types:
nonrecirculating and recirculating. Nonrecirculating systems, also termed
"one-pass" or "single-pass" systems, take in fresh air from
the outside and circulate filtered and conditioned air (i.e., controlled for temperature
and humidity) through the room. Whatever volumes of fresh air are introduced into the room
are ultimately exhausted to the outside. Waste anesthetic gases can be efficiently
disposed of via this nonrecirculating system.
When a nonrecirculating ventilation system serves through large-diameter
tubing and terminating the tubing at the room’s ventilation exhaust as
the disposal route for excess anesthetic gases, disposal involves directing the waste
gases grille. The sweeping effect of the air flowing into the grille carries the waste
gases away. Because all of the exhausted air is vented to the external atmosphere in this
type of system, the excess anesthetic gases can be deposited into the exhaust stream
either at the exhaust grille or further downstream in the exhaust duct.
Concern for fuel economy has increased the use of systems that recirculate air.
Recirculating HVAC/ventilation systems return part of the exhaust air back
into the air intake and recirculate the mixture through the room. Thus, only a fraction of
the exhaust air is disposed of to the outside. To maintain minimal levels of anesthetic
exposure, air which is to be recirculated must not contain anesthetic gases. Consequently,
recirculating systems employed as a disposal pathway for waste anesthetic gases must not
be used for gas waste disposal. The exception is an arrangement that transfers waste gases
into the ventilation system at a safe distance downstream from the point of recirculation
to ensure that the anesthetic gases will not be circulated elsewhere within the building.
Under certain circumstances a separate duct for venting anesthetic gases directly
outside the building without the use of a fan, may be an acceptable alternative. By this
technique, excess anesthetic gases may be vented through the wall, window, ceiling, or
floor, relying only on the slight positive pressure of the gases leaving the gas
collection assembly to provide the flow. However, several limitations are apparent. A
separate line would be required for each OR to prevent the
cross-contamination with anesthetic gases among the ORs. A safe disposal
site would be necessary. The possible effects of variations in wind velocity and direction
would require a means for preventing a reverse flow in the disposal system. Occlusion of
the outer portion of such a passive system by ice or by insect or bird nests is also
possible. The outside opening of a through-wall, -window, -ceiling, or
-floor disposal assembly should be directed downward, shielded, and screened to prevent
the entrance of foreign matter or ice buildup. Despite these limitations, the separate
duct without the use of a fan may be ideal in older facilities constructed with windows
that cannot be opened and in the absence of nonrecirculating air conditioning.
Adsorbers can also trap most excess anesthetic gases. Canisters of varying
shapes and capacities filled with activated charcoal have been used as waste gas disposal
assemblies by directing the gases from the gas disposal tubing through them. Activated
charcoal canisters will effectively adsorb the vapors of halogenated anesthetics but not
N2O. The effectiveness of individual canisters and various
brands of charcoal vary widely. Different potent inhaled volatile agents are adsorbed with
varying efficiencies. The efficiency of adsorption also depends on the rate of gas flow
through the canister. The canister is used where portability is necessary. The disadvantages
are that they are expensive and must be changed frequently. Canisters must be used and
discarded in the appropriate manner, as recommended by the manufacturer.
General or Dilution Ventilation
An effective room HVAC system when used in combination with an anesthetic gas scavenging
system should reduce, although not entirely eliminate, the contaminating
anesthetic gases. If excessive concentrations of anesthetic gases are present, then
airflow should be increased in the room to allow for more air mixing and further dilution
of the anesthetic gases. Supply register louvers located in the ceiling should be designed
to direct the fresh air toward the floor and toward the health-care workers
to provide dilution, and removal of the contaminated air from the operatory or PACU.
Exhaust register louvers should be properly located (usually low on the wall near the
floor level) in the room to provide adequate air distribution. They should not be located
near the supply air vents because this will short-circuit the airflow and
prevent proper air mixing and flushing of the contaminants from the room.
- Work Practices
Work practices, as distinct from engineering controls, involve the way in which a task
is performed. OSHA has found that appropriate work practices can be a vital aid in
reducing the exposures of OR personnel to waste anesthetic agents. In contrast, improper
anesthetizing techniques can contribute to increased waste gas levels. These techniques
can include an improperly selected and fitted face mask, an insufficiently inflated
tracheal tube cuff, an improperly positioned laryngeal mask, or other airway, and careless
filling of vaporizers and spillage of liquid anesthetic agents.
General work practices recommended for anesthetizing locations include the following:
- A complete anesthesia apparatus checkout procedure should be performed each day
before the first case. An abbreviated version should be performed before each
subsequent case. The FDA Anesthesia Apparatus Checkout Recommendations (Appendix 2) should be considered in developing inspection and
testing procedures for equipment checkout prior to administering an anesthetic.
- If a face mask is to be used for administration of inhaled anesthetics, it
should be available in a variety of sizes to fit each patient properly. The mask
should be pliable and provide as effective a seal as possible against leakage into the surrounding air.
- Tracheal tubes, laryngeal masks, and other airway devices should be positioned
precisely and the cuffs inflated adequately.
- Vaporizers should be filled in a well-ventilated area and in a
manner to minimize spillage of the liquid agent. This can be accomplished by using a
specialized "key-fill" spout to pour the anesthetic into the vaporizer
instead of pouring from a bottle into a funnel-fill vaporizer. When
feasible, vaporizers should be filled at the location where the anesthetic will be
administered and, when filled electively, with the fewest possible personnel present
in the room. Vaporizers should be turned off when not in use.
- Spills of liquid anesthetic agents should be cleaned up promptly. (Refer to
section G - Clean-up and
Disposal of Liquid Anesthetic Agent Spills.)
- Before extubating the patient's trachea or removing the mask or other airway
management device, one should administer non-anesthetic gases/agents so
that the washed-out anesthetic gases can be removed by the scavenging
system. The amount of time allowed for this should be based on clinical assessment and
may vary from patient to patient. When possible, flushing of the breathing system
should be achieved by exhausting into the scavenging system rather than into the room air.
Work practices performed by biomedical engineers and technicians also contribute
significantly to the efficacy of managing waste gas exposure. It is, therefore, important
for this group of workers to do the following:
- Monitor airborne concentrations of waste gases by sampling, measuring, and
reporting data to the institution's administration. Air monitoring for waste
anesthetic gases should include both personal sampling (i.e., in a
health-care worker’s breathing zone) and area sampling.
- Assist in identifying sources of waste/leaking gases and implementing corrective action.
- Determine if the scavenging system is designed and functioning properly to
remove the waste anesthetic gases from the breathing circuit, and ensure that the
gases are vented from the workplace in such a manner that occupational
re-exposure does not occur (e.g., smoke trail tests of exhaust grilles
used with passive scavenging systems).
- Ensure that operatory and PACU ventilation systems provide sufficient room air
exchange to reduce ambient waste gas levels.
- Administrative Controls
Administrative controls represent another approach for reducing worker exposure to
waste gases other than through the use of engineering controls, work practices, or
personal protective equipment. Administrative controls may be thought of as any
administrative decision that results in decreased anesthetic-gas exposure.
For workers potentially exposed to waste anesthetic gases, the program administrator
should establish and implement policies and procedures to:
- Institute a program of routine inspection and regular maintenance of equipment
in order to reduce anesthetic gas leaks and to have the best performance of scavenging
equipment and room ventilation. Preventive maintenance should be performed by trained
individuals according to the manufacturer’s recommendations and at intervals
determined by equipment history and frequency of use. Preventive maintenance includes
inspection, testing, cleaning, lubrication, and adjustment of various components. Worn
or damaged parts should be repaired or replaced. Such maintenance can result in
detection of deterioration before an overt malfunction occurs. Documentation of the
maintenance program should be kept indicating the nature and date of the work
performed, as well as the name of the trained individual servicing the equipment.
- Implement a monitoring program to measure airborne levels of waste gases in the
breathing zone or immediate work area of those most heavily exposed (e.g.,
anesthesiologist, nurse anesthetist, oral surgeon) in each anesthetizing location and
PACU. Periodic monitoring (preferably at least semiannually) of waste gas
concentrations is needed to ensure that the anesthesia delivery equipment and
engineering/environmental controls work properly and that the maintenance program is
effective. Monitoring may be performed effectively using conventional
time-weighted average air sampling or real-time air sampling techniques.
- Encourage or promote the use of scavenging systems in all anesthetizing
locations where inhaled agents are used, recognizing that a waste gas scavenging
system is the most effective means of controlling waste anesthetic gases.
- Implement an information and training program for employees exposed to
anesthetic agents that complies with OSHA’s Hazard Communication Standard (29 CFR
1910.1200) so that
employees can meaningfully participate in, and support, the protective measures
instituted in their workplace.
- Define and implement appropriate work practices to help reduce employee
exposure. Training and educational programs covering appropriate work practices to
minimize levels of anesthetic gases in the operating room should be conducted at least
annually. Employers should emphasize the importance of implementing these practices
and should ensure that employees are properly using the appropriate techniques on a
regular basis.
- Implement a medical surveillance program for all workers exposed to waste gases.
- Ensure the proper use of personal protective equipment during
clean-up and containment of major spills of liquid anesthetic agents.
- Manage disposal of liquid agents, spill containment, and air monitoring for
waste gases following a spill.
- Comply with existing federal, state, and local regulations and guidelines
developed to minimize personnel exposure to waste anesthetic gases, including the
proper disposal of hazardous chemicals.
- Personal Protective Equipment
Personal protective equipment should not be used as a substitute for engineering, work
practice, and/or administrative controls in anesthetizing locations and PACUs. In fact,
exposure to waste gases is not effectively reduced by gloves, goggles, and surgical masks.
A negative-pressure, high-efficiency particulate air (HEPA) filter used for
infection control is also not appropriate to protect workers from waste gases.
Air-supplied respirators with self-contained air source are
ideal for eliminating exposure but are not a practical alternative.
During clean-up and containment of spills of liquid anesthetic agents,
personal protective equipment should be used in conjunction with engineering, work
practice, and/or administrative controls to provide for employee safety and health.
Gloves, goggles, face shields, and chemical protective clothing (CPC) are recommended to
ensure worker protection. Respirators, where needed, should be selected based on the
anticipated contamination level.
When selecting gloves and CPC, some of the factors to be considered include material
chemical resistance, physical strength and durability, and overall product integrity.
Permeation, penetration, and degradation data should be consulted if available. Among the
most effective types of gloves and body protection are those made from
Viton®, neoprene, and nitrile. Polyvinyl alcohol (PVA) is
also effective but it should not be exposed to water or aqueous solutions.
When the gloves and the CPC being used have not been tested under the expected
conditions, they may fail to provide adequate protection. In this situation, the wearer
should observe the gloves and the chemical protective clothing during use and treat any
noticeable change (e.g., color, stiffness, chemical odor inside) as a failure until proved
otherwise by testing. If the work must continue, new CPC should be worn for a shorter
exposure time, or CPC of a different generic material should be worn. The same thickness
of a generic material such as neoprene or nitrile supplied by different manufacturers may
provide significantly different levels of protection because of variations in the
manufacturing processes or in the raw materials and additives used in processing.
Professional judgement must be used in determining the type of respiratory protection
to be worn. For example, where spills of halogenated anesthetic agents are small, exposure
time brief, and sufficient ventilation present, NIOSH-approved chemical
cartridge respirators for organic vapors should provide adequate protection during cleanup
activities.
Where large spills occur and there is insufficient ventilation to adequately reduce
airborne levels of the halogenated agent, respirators designed for increased respiratory
protection should be used. The following respirators, to be selected for large spills, are
ranked in order from minimum to maximum respiratory protection:
- Any type 'C' supplied-air respirator with a full facepiece,
helmet, or hood operated in continuous-flow mode.
- Any type 'C' supplied-air respirator with a full facepiece
operated in pressure-demand or other positive-pressure mode.
- Any self-contained breathing apparatus with a full facepiece
operated in pressure-demand or other positive-pressure mode.
F. LOCATION-SPECIFIC WORKPLACE CONTROLS
This section describes engineering and work practice controls specific to hospital ORs,
PACUs, dental operatories, and veterinary clinics and hospitals. Operational procedures
relating to engineering controls are also discussed where appropriate.
- Hospital Operating Rooms
For years anesthesia providers tolerated exposure to waste anesthetic gases and
regarded it as an inevitable consequence of their work. Since the 1970s anesthesiologists
have steadily worked to improve equipment and technique to reduce workplace exposures to
waste anesthetic gases, and significant progress has been made. In early delivery
equipment, waste gases were exhausted through the APL or "pop-off" valve
into the face of the anesthesia provider and were distributed into the room air. Present
practice which utilizes an efficient scavenging system avoids this type of contamination
by collecting the excess gases immediately at the APL valve.
- Engineering Controls
Waste gas evacuation is required for every type of breathing circuit configuration
(Huffman 1991; Azar and Eisenkraft 1993), with the
possible exception of a closed circuit, because most anesthesia techniques typically use
more fresh gas flow than is required. Appropriate waste gas evacuation involves collection
and removal of waste gases, detection and correction of leaks, consideration of work
practices, and effective room ventilation (Dorsch and Dorsch 1994). To
minimize waste anesthetic gas concentrations in the operating room the recommended air
exchange rate (room dilution ventilation) is a minimum total of 15 air changes per hour
with a minimum of 3 air changes of outdoor air (fresh air) per hour (American Institute
of Architects 1996-1997). Operating room air containing waste anesthetic
gases should not be recirculated to the operating room or other hospital locations.
- Work Practices
In most patients, a circle absorption system is used and can be easily connected to a
waste gas scavenging system. In pediatric anesthesia, systems other than those with a
circle absorber may be used. Choice of the breathing circuit that best meets the needs of
pediatric patients may alter a clinician’s ability to scavenge waste gas effectively.
Breathing circuits frequently chosen for neonates, infants, and small children are usually
valveless, have low resistance, and limit rebreathing. The Mapleson D system and the
Jackson-Rees modification of the Ayre’s T-piece are examples of
limited rebreathing systems that require appropriate scavenging equipment.
The following work practices may be employed with any of the above breathing circuits:
- Empty the contents of the reservoir bag directly into the anesthetic gas
scavenging system and turn off the flow of N2O and
any halogenated anesthetic agent prior to disconnecting the patient circuit.
- Turn off the flow of N2O and the vaporizer, if
appropriate, when the patient circuit is disconnected from the patient, for example,
for oral or tracheal suctioning.
- Test daily for low-pressure leaks throughout the entire anesthesia
system. All leaks should be minimized before the system is used. Starting anesthetic
gas flow before the actual induction of anesthesia begins is not acceptable. For
techniques to rapidly induce anesthesia using inhaled agents
(single-breath mask induction), the patient connector should be occluded
when filling the breathing circuit with nitrous oxide or halogenated agent prior to
applying the mask to the patient's face.
If the circle absorber system (Figure 6) is used, the following additional work practices can be employed:
- Adjust the vacuum needle valve as needed to regulate the flow of waste anesthetic gases into the vacuum
source in an active scavenging system. Adjustments prevent the bag from overdistending by maintaining the volume
in the scavenging system reservoir bag between empty and half-full (Bowie and Huffman 1985;
Huffman 1991). In machines that use an open reservoir to receive waste
gas, a flowmeter is used to adjust the rate of gas flow to the vacuum system.
- Cap any unused port in a passive waste gas scavenging configuration.
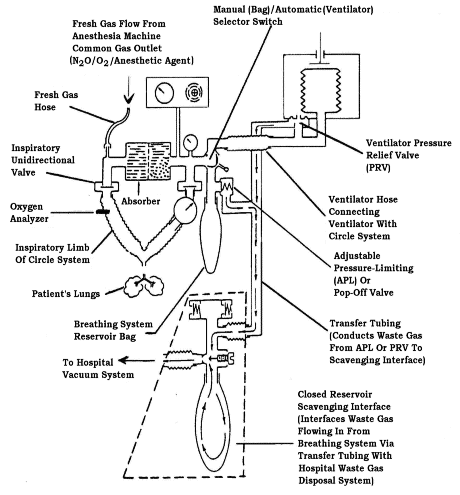
Figure 6
Circle breathing system connected to a closed reservoir scavenging interface.
(Reproduced by permission of North American Dräger, Telford, Pennsylvania).
|
- Postanesthesia Care in Hospitals and Stand-Alone Facilities
Because the patient is the main source of waste anesthetic gases in the PACU, it
becomes more difficult to control health-care workers’ exposures to waste
anesthetic gases. The unique PACU environment coupled with the patient’s
immediate condition upon arrival from surgery require different work
practices than those routinely used in ORs. Patients undergoing general
anesthesia usually have their airways secured using a tracheal tube with
an inflatable cuff that seals the tube within the trachea. The seal
between the tracheal tube cuff and the trachea (or between the face mask
and the face) is essential for maintaining a gas-tight system that permits effective
scavenging in the OR. The tracheal tube connects the patient with the
breathing circuit which is connected to the scavenging system in the OR.
Once the patient reaches the PACU, scavenging systems such as those used
in the OR are no longer effective, since the patient is no longer
connected to the breathing circuit. Other less-effective methods of waste gas removal
are thus relied upon.
- Engineering Controls
As a result of using appropriate anesthetic gas scavenging in ORs, the levels of contamination have been decreased. In the PACU, however,
the principle of scavenging as practiced in the OR is not widely accepted due to medical considerations and consequently is infrequently
employed as a source-control method for preventing the release of waste anesthetic gases into the PACU environment. Most
PACUs provide care to multiple patients in beds without walls between them, and convective currents move the gases from their source to
other areas. Therefore, in the PACU, a properly designed and operating dilution ventilation system should be relied upon to minimize
waste anesthetic gas concentrations. This system should provide a recommended minimum total of 6 air changes per hour with a minimum of
2 air changes of outdoor air per hour to adequately dilute waste anesthetic gases
(American Institute of Architects 1996-1997). Room exhaust
containing waste anesthetic gases should not be recirculated to other areas of the hospital.
- Work Practices
PACU managers should consider:
- Periodic exposure monitoring with particular emphasis on peak gas levels in the breathing zone of
nursing personnel working in the immediate vicinity of the patient’s head. Methods using random room sampling to
assess ambient concentrations of waste anesthetic gases in the PACU are not an accurate indicator of the level of
exposure experienced by nurses providing bedside care. Because of the closeness of the PACU nurse to the patient,
such methods would consistently underestimate the level of waste anesthetic gases in the breathing zone of the
bedside nurse.
- Application of a routine ventilation system maintenance program to keep waste gas exposure levels to a minimum.
- Dental Operatory
Mixtures of N2O and oxygen have been used in
dentistry as general anesthetic agents, analgesics, and sedatives for
more than 100 years (McGlothlin et al. 1992). The usual analgesia
equipment used by dentists includes a N2O and O2 delivery
system, a gas mixing bag, and a nasal mask with a positive pressure
relief valve (Dorsch and Dorsch 1994). The analgesia machine
is usually adjusted to deliver more of the analgesic gas mixture than
the patient can use.
Analgesia machines for dentistry are designed to deliver up to 70 percent (700,000 ppm)
N2O to a patient during dental surgery. The machine restricts higher concentrations of
N2O from being administered to protect the patient from hypoxia. In most cases, patients
receive between 30 and 50 percent N2O during surgery. The amount of time
N2O is administered to a patient depends on the dentist’s judgment of patient needs and the
complexity of the surgery. The most common route of N2O delivery and exhaust is through
a nasal scavenging mask applied to the patient.
Some dentists administer N2O at
higher concentrations at the beginning of the operation, then decrease
the amount as the operation progresses. Others administer the same
amount of N2O throughout the operation. When the
operation is completed, the N2O is turned off. Some dentists turn
the N2O on only at the beginning of the
operation, using N2O as a sedative during the
administration of local anesthesia, and turn it off before operating
procedures. Based on variations in dental practices and other factors in
room air, N2O concentrations can vary
considerably for each operation and also vary over the course of the operation.
Unless the procedure is performed under general anesthesia in an OR, halogenated anesthetics are not administered,
nor does the patient undergo laryngoscopy and tracheal intubation. In the typical dental office procedure, the nasal
mask is placed on the patient, fitted, and adjusted prior to administration of the anesthetic agent. The mask is
designed for the nose of the patient since access to the patient’s mouth is essential for dental procedures.
A local anesthetic, if needed, is typically administered after the N2O takes effect. The
patient’s mouth is opened and the local anesthetic is injected. The dental procedure begins after the local anesthetic
takes effect. The patient opens his/her mouth but is instructed to breathe through the nose. Nonetheless, a certain
amount of mouth breathing frequently occurs. The dentist may periodically stop the dental procedure for a moment to
allow the patient to close the mouth and breath deeply to re-establish an appropriate concentration of
N2O in the patient’s body before resuming the procedure. Depending on the nature of the
procedure, high velocity suction is regularly used to remove intraoral debris and, when used, creates a negative air
flow and captures some of the gas exhaled by the patient.
At the end of the procedure, the nosepiece is left on the patient while the N2O is turned
off and the oxygen flow is increased. The anesthetic mixture diffuses from the circulating blood into the lungs and is
exhaled. Scavenging is continued while the patient is eliminating the N2O.
- Engineering Controls
The dental office or operatory should have a properly installed N2O delivery system.
This includes appropriate scavenging equipment with a readily visible and accurate flow meter (or equivalent measuring
device), a vacuum pump with the capacity for up to 45 L/min of air per workstation, and a variety of sizes of masks to
ensure proper fit for individual patients.
A common nasal mask, shown in Figure 7, consists of an inner and a slightly larger outer mask component. The inner
mask has two hoses connected that supply anesthetic gas to the patient. A relief valve is attached to the inner mask
to release excess N2O into the outer mask. The outer mask has two smaller hoses connected to
a vacuum system to capture waste gases from the patient and excess gas supplied to the patient by the analgesia
machine. The nasal mask should fit over the patient’s nose as snugly as possible without impairing the vision or
dexterity of the dentist. Gases exhaled orally are not captured by the nasal mask. A flow rate of approximately 45
L/min has been recommended as the optimum rate to prevent significant N2O leakage into the
room air (NIOSH 1994).

Figure 7
Circle breathing system connected to a closed reservoir scavenging interface. (Reproduced
by permission of North American Dräger, Telford, Pennsylvania).
|
A newer type of mask is a frequent choice in dental practice: a single patient use nasal hood. This mask does not
require sterilization after surgery because it is used by only one patient and is disposable.
In a dental operatory, a scavenging system is part of a high-volume evacuation system used with a dental unit.
The vacuum system may dispose of a combination of waste gases, oral fluid, and debris, and is not limited to waste gas removal. The
exhaust air of the evacuation system should be vented outside the building and away from fresh-air inlets and open
windows to prevent re-entry of gas into the operatory.
The general ventilation should provide good room air mixing. In addition, auxiliary (local) exhaust ventilation used in conjunction
with a scavenging system has been shown to be effective in reducing excess N2O in the breathing zone of the dentist and
dental assistant, from nasal mask leakage and patient mouth breathing (NIOSH 1994). This type of
ventilation captures the waste anesthetic gases at their source. However, there are practical limitations in using it in the dental
operatory. These include proximity to the patient, interference with dental practices, noise, and installation and maintenance costs. It
is most important that the dentist not work between the patient and a free-standing local exhaust hood. Doing so will
cause the contaminated air to be drawn through the dentist’s breathing zone. These auxiliary ventilation systems are not now commercially
available.
- Work Practices
- Prior to first use each day of the N2O machine and every time a gas cylinder
is changed, the low-pressure connections should be tested for leaks. High-pressure
line connections should be tested for leaks quarterly. A soap solution may be used to test for leaks at
connections. Alternatively, a portable infrared spectrophotometer can be used to detect an insidious leak.
- Prior to first use each day, inspect all N2O equipment (e.g., reservoir bag,
tubing, mask, connectors) for worn parts, cracks, holes, or tears. Replace as necessary.
- Connect mask to the tubing and turn on vacuum pump. Verify appropriate flow rate (i.e., up to 45 L/min or
manufacturer’s recommendations).
- A properly sized mask should be selected and placed on the patient. A good, comfortable fit should be
ensured. The reservoir (breathing) bag should not be over- or underinflated while the patient is
breathing oxygen (before administering N2O).
- Encourage the patient to minimize talking, mouth breathing, and facial movement while the mask is in place.
- During N2O administration, the reservoir bag should be periodically inspected
for changes in tidal volume, and the vacuum flow rate should be verified.
- On completing anesthetic administration and before removing the mask, non-anesthetic
gases/agents should be delivered to the patient for a sufficient time based on clinical assessment that may vary
from patient to patient. In this way, both the patient and the system will be purged of residual
N2O. Do not use an oxygen flush.
- Veterinary Clinics and Hospitals
Inhalation anesthesia in veterinary hospitals is practiced in a manner similar to that in human hospitals.
Generally, animals are initially given an injectable anesthetic, followed by general anesthesia maintained by an
inhalation technique. In animal anesthesia, there are five basic methods by which inhalation anesthetics
are administered: open-insufflation, semiopen without nonrebreathing valves, semiopen with nonrebreathing
valves, semiclosed, and closed. Figure 8 illustrates a circle breathing system. Oxygen and anesthetic are transported
to the animal’s lungs from the anesthesia machine through a face mask or tracheal tube. An inflatable cuff on the
distal end of the tracheal tube facilitates a seal with the inner wall of the trachea.
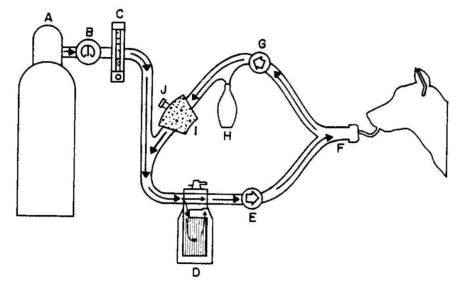
Figure 8
Circle breathing system used for veterinary anesthesia.
(Reproduced by permission of
American Industrial Hygiene Association, Fairfax, Virginia).
|
Unidirectional valves allow flow from the vaporizer to the animal upon inspiration and route the exhaled gases
through a carbon dioxide absorber during expiration. High fresh-gas flows are typically used with all
techniques except closed-system breathing circuits. During expiration, excess or waste gas exits the
breathing circuit at the adjustable pressure-limiting (APL) or pop-off valve and escapes
into the room unless it is appropriately scavenged.
Non-rebreathing systems allow exhaled gases to be immediately expelled from the
system into the room air. Because these systems do not include a carbon
dioxide absorber, greater fresh-gas
flows are required to ensure removal of carbon dioxide from the system.
A higher fresh-gas flow may lead to an
increase in ambient waste gas levels.
- Engineering Controls
The basic principles of scavenging used to capture excess anesthetic gases in hospital surgical suites are
appropriate for application in veterinary anesthesia. The APL or pop-off valve is connected to the
scavenging interface valve. A waste gas reservoir bag is attached to the interface valve and collects excess
anesthetic gases.
In general, the disposal pathway for waste anesthetic gases generated in a veterinary facility can be any one
of those mentioned (e.g., ventilation system, central vacuum system, dedicated blower [exhaust] system, passive
duct system, or adsorber) and described in detail on pages [15-17] of this
document. A vacuum source, if present, is connected to the interface valve and waste gas reservoir bag, where gas
is stored until the vacuum can move it to the outside air. If only halogenated compounds are used, an activated
charcoal adsorption system can be used.
- Work Practices
The following are recommended work practices for reducing gas leakage:
- Avoid turning on N2O or a vaporizer until the circuit is connected to the animal. Switch off the
N2O and vaporizer when not in use. Maintain oxygen flow until the scavenging system is flushed.
- Select the optimal size tracheal tube for the animal and make sure the cuff, if present, is adequately
inflated. Adequacy of cuff inflation may be evaluated by delivering a positive-pressure breath while
the APL or pop-off valve is closed and listening for a leak originating from around the tracheal
tube cuff.
- Occlude the Y-piece if the breathing circuit must be disconnected during surgery.
- Once anesthesia is discontinued, empty the breathing bag into the scavenging system rather than into the
room. Releasing anesthetic gases into the OR could significantly increase the overall waste gas concentration
within the room.
- At the end of the surgical procedure, continue to administernon-anesthetic gases/agents as
long as clinically necessary, using high oxygen flow rates through the breathing circuit to wash the anesthetic
gases out of the system and the animal. This allows exhaled anesthetic gases to be collected by the scavenging
system.
- It is possible to close an anesthetic circle and reduce fresh-gas flow rates. In a circle
system where oxygen is the only carrier gas, the amount of fresh gas flowing to the animal should be adjusted to
closely match the animal’s metabolic oxygen requirement.
- Select masks to suit various sizes and breeds encountered in veterinary practice. When a mask is used for
induction or maintenance of anesthesia, use a mask that properly fits the contour of the animal’s face to
minimize gas leakage. Minimize the time of mask anesthesia to reduce waste.
- Use a box for induction of anesthesia in small, uncooperative animals. As with the mask technique, the
induction box method requires high gas-flow rates, with substantial anesthetic spillage. Methods to
minimize this spillage include tight seals on the box and placement of the box near the ventilation port of a
well-ventilated room. The box can also be connected to an anesthetic gas-scavenging
system to evacuate the gases in the box prior to removing the animal.
- Make certain that the reservoir bag, used to store excess anesthetic waste gas until the vacuum system can
remove it, is adequate to contain all scavenged gas. This reservoir bag is especially designed to connect to
anesthetic gas-specific fittings.
G. CLEAN-UP AND DISPOSAL OF LIQUID ANESTHETIC AGENT SPILLS
Small volumes of liquid anesthetic agents such as halothane, enflurane, isoflurane, desflurane, and sevoflurane
evaporate readily at normal room temperatures, and may dissipate before any attempts to clean up or collect the
liquid are initiated. However, when large spills occur, such as when one or more bottles of a liquid agent break,
specific cleaning and containment procedures are necessary and appropriate disposal is required (AANA 1992).
The recommendations of the chemical manufacturer’s material safety data sheet (MSDS) that identify
exposure reduction techniques for spills and emergencies should be followed.
In addition, OSHA Standard for Hazardous Waste Operations and Emergency Response (29 CFR 1910.120) would apply if
emergency response efforts are performed by employees. The employer must determine the potential for an emergency in a reasonably
predictable worst-case scenario, and plan response procedures accordingly. Only adequately trained and equipped
workers may respond to spills. When the situation is unclear or data are lacking on the exposure level, the response needs
to be the same as for high levels of exposure. Responses to incidental releases of liquid anesthetic agents where the
substance can be absorbed, neutralized, or otherwise controlled at the time of release by employees in the immediate
release area, or by maintenance personnel do not fall within the scope of this standard.
Because of the volatility of liquid anesthetics, rapid removal by suctioning in the OR is the preferred method for cleaning
up spills. Spills of large volumes in poorly ventilated areas or in storage areas should be absorbed using an absorbent material,
sometimes called a sorbent, that is designed for clean-up of organic chemicals. "Spill pillows" commonly used in hospital
laboratories, vermiculite, and carbon-based sorbents are some of the materials commercially available and regularly
used for this purpose. Caution should be exercised if broken glass bottles pose a hazard.
Both enflurane and desflurane are considered hazardous wastes under the EPA regulations because these chemicals contain
trace amounts of chloroform (a hazardous substance), a by-product of the manufacturing process.Consequently, sorbents
that have been saturated with enflurane or desflurane should be managed as an EPA hazardous waste material due to the trace
concentrations of chloroform present. Isoflurane and halothane do not contain trace amounts of chloroform or any other regulated
substance and are therefore not considered hazardous wastes by EPA.
To minimize exposure to all liquid anesthetic agents during clean-up and to limit exposure during disposal procedures,
the following general guidelines are recommended. The waste material should be placed in a container, tightly sealed, properly
labeled, and disposed of with other chemical wastes sent to a facility’s incinerator or removed by a chemical waste contractor.
After a large spill has occurred and the appropriate response action taken, airborne monitoring should be conducted to determine
if the spill was effectively contained and cleaned up.
Determination of appropriate disposal procedures for each facility is the sole responsibility of that facility. Empty anesthetic
bottles are not considered regulated waste and may be discarded with ordinary trash or recycled. Furthermore, the facility as well as
the waste handling contractor must comply with all applicable federal, state, and local regulations.
To minimize exposure to waste liquid anesthetic agents during clean-up and disposal, the following general guidelines
are recommended by the manufacturers of liquid anesthetic agents:
- Wear appropriate personal protective equipment. (Refer to section E4 on
personal protective equipment).
- Where possible, ventilate area of spill or leak. Appropriate respirators should be worn.
- Restrict persons not wearing protective equipment from areas of spills or leaks until clean-up is complete.
- Collect the liquid spilled and the absorbent materials used to contain a spill in a glass or plastic container. Tightly cap
and seal the container and remove it from the anesthetizing location. Label the container clearly to indicate its contents.
- Transfer the sealed containers to the waste disposal company that handles and hauls waste materials.
- Health-care facilities that own or operate medical waste incinerators may dispose of waste anesthetics by using an appropriate
incineration method after verifying that individual incineration operating permits allow burning of anesthetic agents at each site.
H. AIR MONITORING
Air monitoring is one of the fundamental tools used to evaluate workplace exposures. Accordingly, this section
presents some of the appropriate methods that can be used to detect and measure the concentration of anesthetic gases
that may be present in the health-care environment. The data provided by monitoring are necessary to
establish proper engineering, work practice, and administrative controls to ensure the lowest reasonably achievable
gas levels in the operatory and PACU room air.
OSHA recommends that air sampling for anesthetic gases be conducted every 6 months to measure worker exposures and
to check the effectiveness of control measures. Furthermore, OSHA recommends that only the agent(s) most frequently
used needs to be monitored, since proper engineering controls, work practices and control procedures should reduce all
agents proportionately. However, the decision to monitor only selected agents could depend not only on the frequency
of their use, but on the availability of an appropriate analytical method and the cost of instrumentation. [ASA
emphasizes regular maintenance of equipment and scavenging systems, daily check-out procedures for
anesthesia equipment, and education to ensure use of appropriate work practices. It does not believe that a routine
monitoring program is necessary when these actions are being carried out. ASA prefers to use monitoring when indicated
such as in the event of known or suspected equipment malfunction. The Academy of General Dentistry also emphasizes
properly installed and maintained analgesia delivery systems.]
Three fundamental types of air samples can be taken in order to evaluate the workplace: personal, area, and source
samples. Personal samples give the best estimate of a worker’s exposure level since they represent the actual airborne
contaminant concentration in the worker’s breathing zone during the sampling period. This is the preferred method for
determining a worker’s time-weighted average (TWA) exposure and should be used to assess personal
exposures during anesthetic administration and in the PACU. Where several health-care workers perform the
same job, on the same shift, and in the same work area, and the length, duration, and level of waste gas exposures are
similar, an employer may sample a representative fraction of the employees instead of all employees.
Area sampling is useful for evaluating overall air contaminant levels in a work area and for investigating
cross-contamination with other areas in the health-care facility. Source sampling can be
used to detect leaks in the anesthesia delivery and scavenging systems as well as ineffective capture by the
scavenging system. Thus, how samples are taken is a critical point in any safety program.
The OSHA Chemical Information Manual contains current sampling technology for several of the anesthetic
gases that may be present in anesthetizing locations and PACUs. Some of the sampling methods available are summarized
below.
- Time-Integrated Sampling
- Nitrous Oxide
Personal N2O exposures can be determined by using the VAPOR-TRAK nitrous oxide
passive monitor (sometimes called a"passive dosimeter" or"diffusive sampler") as referenced in the 2000 OSHA
Chemical Information Manual under IMIS:1953. The minimum sampling duration for the dosimeter is 15 minutes;
however, it can be used for up to 16 hours of passive sampling. This sampler has not been validated by OSHA. Other
dosimeters are commercially available and can be used. Although not validated by OSHA at this time, they may be
validated in the future. Five liter, 5-layer aluminized gas sampling bags can also be used to collect a
sample.
- Halogenated Agents
Three chlorofluorocarbon-based anesthetic agents (halothane, enflurane, and isoflurane) and one
fluorocarbon-based agent (desflurane) are listed in the Chemical Information Manual. The OSHA
sampling procedure for halothane is listed under IMIS:0395; for enflurane, under IMIS:1038; for isoflurane, under
IMIS:F118; and for desflurane, under IMIS:R218.
The current recommended media sampling for halothane, enflurane, and isoflurane requires an Anasorb 747 tube
(140/70 mg sections) or an Anasorb CMS tube (150/75 mg. sections). The sample can be taken at a flow rate of 0.5
L/min. Total sample volumes not exceeding 12 liters are recommended. The current recommended sampling media for
desflurane requires an Anasorb 747 tube (140/70 mg sections). The sample can be taken at a flow rate of 0.05
L/min. Total sample volumes not exceeding 3 liters are recommended. All four sampling methodologies are fully
validated analytical procedures.
- Real-Time Sampling
Sampling that provides direct, immediate, and continuous (real-time) readout of anesthetic gas concentrations in
ambient air utilizes a portable infrared spectrophotometer. Since this method provides continuous sampling and
instantaneous feedback, sources of anesthetic gas leakage and effectiveness of control measures can be immediately
determined.
- Additional Sampling Guidelines
If it should ever be necessary to enter an operating room to conduct air sampling, the following guidelines
provide the information needed. Individuals performing air sampling should be familiar with and follow all OR
procedures for access into and out of the surgical suite with particular attention to sterile and nonsterile
areas. The patient is the center of the sterile field, which includes the areas of the patient, operating table, and
furniture covered with sterile drapes and the personnel wearing sterile attire. Sampling in the breathing zone of
surgeons and other nursing or technical personnel who work in the sterile field must conform to the principles of
sterile field access. Strict adherence to sound principles of sterile technique and recommended practices is mandatory
for the safety of the patient.
Generally speaking, each hospital has its own
guidelines for proper OR attire and other safety procedures. These rules
should be strictly followed by anyone entering the OR. There are
standard uniform guidelines that apply to all hospitals. Only clean
and/or freshly laundered OR attire is worn in the OR. Proper attire
consists of body covers such as a two-piece pantsuit (scrub suit), head cover
(cap or hood), mask, and shoe covers. A sterile gown is worn over the
scrub suit to permit the wearer to come within the sterile field. Other
attire such as gloves and eyewear may be required. Some hospitals, but
not all, may allow persons coming into the OR to wear a clean gown (in
addition to the cap, the mask, and the shoe covers) over their street
clothes if they are not going to remain in the OR for longer than 10-15 minutes.
In regard to decontaminating outside equipment,
each hospital has its own policy. However, the common practice is to
"wipe off" all surfaces with a chemical disinfectant. Most hospitals use
Wescodyne or other phenolic solutions. Good physical cleaning before
disinfection helps reduce the number of microorganisms present and
enhances biocidal action.
Any person not familiar with the OR is usually
instructed by a scrub nurse on all the safety procedures pertaining to
the hospital. The scrub nurse will also provide instructions on hand
scrubbing and other procedures that may be necessary. Persons entering
the OR must follow these guidelines and instructions.
In addition, it should be recognized that the patient’s welfare, safety, and rights of privacy are
paramount.
I. MEDICAL SURVEILLANCE
In all locations where anesthesia is administered, engineering controls such as a scavenging system to remove waste
anesthetic gases and adequate room ventilation should be utilized. Medical surveillance of personnel working in
scavenged operating rooms is intended primarily to establish a baseline. Routine annual follow-up is
primarily educational and at minimum, might consist of a health questionnaire. Examinations and laboratory testing
should be available for conditions suspected of being related to occupational exposure. A sample program might
include:
- A preplacement medical questionnaire that includes a detailed work history (including past exposures to
waste anesthetic gases); a medical history with emphasis on: hepatic (liver), renal (kidney), neurological
(nervous system), cardiovascular (heart and circulation), and reproductive functions. Pertinent positive
response(s) to the questionnaire should be followed by an appropriate medical evaluation (i.e.,
in-depth history and physical examination where appropriate) and, where relevant, suitable
laboratory tests, such as liver function tests.
- An annual questionnaire emphasizing the issues mentioned above. Again, the need for physical examination or
laboratory work may be based on questionnaire responses.
- A system should be created for employees to report health problems which they believe may be associated
with anesthetic exposure. Employees should be informed of this reporting system and of the method by which reports
can be submitted.
- An acute exposure (i.e., a sudden, high-level exposure) should be documented. Any subsequent
health effects should trigger a medical history, and a physical examination (where appropriate).
- A reproductive hazards policy should also be in place at the facility and should address worker exposure
and reproductive health effects in male and female employees. The facility should provide training in the known
and potential adverse health effects, including reproductive effects, of waste anesthetic gases, as is required
for chemicals covered by the Hazard Communication Standard.
- A final medical review upon job transfer or termination. This should be in the form of a questionnaire that
includes any acute or significant exposures as well as a review of symptoms and signs detected during employment,
along with a medical evaluation when appropriate.
- Medical and exposure records developed for employees who may be exposed to hazardous chemicals such as
N2O and halogenated anesthetic agents must be retained, made available, and transferred
in accordance with OSHA Standard for Access to Employee Exposure and Medical Records (29 CFR
1910.1020).
The occurrence of injury or illness related to occupational exposure must be recorded in accordance with OSHA recordkeeping
regulations (29 CFR 1904).
J. HAZARD COMMUNICATION
In accordance with the Hazard Communication Standard (29 CFR
1910.1200),
employers in health-care facilities must develop, implement, and maintain at the workplace a written, comprehensive
hazard communication program that includes provisions for container labeling, collection and availability of material safety data sheets
(MSDSs), and an employee training and information program. The standard also requires a list of hazardous chemicals in
the workplace as part of the written hazard communication program.
Any chemicals subject to the labeling requirements of the FDA are exempt from the labeling requirements under the
Hazard Communication Standard. This includes such chemicals as volatile liquid anesthetics and compressed medical
gases. However, containers of other chemicals not under the jurisdiction of the FDA must be labeled, tagged, or marked
with the identity of the material and must show appropriate hazard warnings as well as the name and address of the
chemical manufacturer, importer, or other responsible party. The hazard warning can be any type of message --words,
pictures, or symbols-- that conveys the hazards of the chemical(s) in the container. Labels must be legible, in
English (plus other languages if desired), and prominently displayed.
Each MSDS must be in English, although the employer may maintain copies in other languages as well, and must
include information regarding the specific chemical identity of the anesthetic gases or hazardous chemical and its
common names. In addition, information must be provided on the physical and chemical characteristics of the hazardous
chemical, known acute and chronic health effects and related health information, primary route(s) of entry, exposure
limits, precautionary measures, emergency and first-aid procedures, and the identification of the
organization responsible for preparing the sheet. As a source of detailed information on hazards, copies of the MSDS
for each hazardous chemical must be readily accessible during each work shift to employees when they are in their work
area(s).
Employers must prepare a list of all hazardous chemicals in the workplace, and the list should be checked to verify
that MSDSs have been received for each chemical. If there are hazardous chemicals used for which no MSDS has been
received, the employer must contact the supplier, manufacturer, or importer to obtain the missing MSDS.
Health-care employers must establish a training and information program for all personnel who are involved in the
handling of, or who have potential exposure to, anesthetic gases and other hazardous chemicals to apprise them of the
hazards associated with these chemicals in the workplace. Training relative to anesthetic gases should place an
emphasis on reproductive risks. Training and information must take place at the time of initial assignment and
whenever a new hazard is introduced into the work area. At a minimum, employees must be informed of the following:
- The Hazard Communication Standard (29 CFR
1910.1200) and its requirements.
- Any operations and equipment in the work area where anesthetic agents and hazardous chemicals are present.
- Location and availability of the written hazard communication program including the required lists of
hazardous chemicals and the required MSDS forms.
The employee training program must consist of the following elements:
- How the hazard communication program is implemented in the workplace, how to read and interpret
information on the MSDS and label of each hazardous chemical, and how employees can obtain and use the
available hazard information.
- The physical and health hazards of the chemicals in the work area.
- Measures employees can take to protect themselves from these hazards, including specific procedures put
into effect by the employer to provide protection such as engineering controls, appropriate work practices,
emergency procedures for spill containment, and the use of personal protective equipment.
- Methods and observations that may be used to detect the presence or release of anesthetic gases and
other hazardous chemicals in the work area (such as monitoring conducted by the employer, continuous
monitoring devices, and the appearance or odor of chemicals when released).
Personnel training records are not required to be maintained, but such records would assist employers in
monitoring their programs to ensure that all employees are appropriately trained. Employers can provide employees
information and training through whatever means are found appropriate and protective. Although there would always
have to be some training on-site (such as informing employees of the location and availability of the
written program and MSDSs), employee training may be satisfied in part by general training about the requirements
of the hazard communication standard and about chemical hazards on the job which is provided by, for example,
professional associations, colleges, universities, and training centers. In addition, previous training, education,
and experience of a worker may relieve the employer of some of the burdens of informing and training that worker.
The employer, however, maintains the responsibility to ensure that their employees are adequately trained and are
equipped with the knowledge and information to do their jobs safely.
K. REFERENCES
American Association of Nurse Anesthetists (AANA). 1992. Management of Waste Anesthetic Gases.
Park Ridge, IL: Author. Pp. 16-17.
American Conference of Governmental Industrial Hygienists (ACGIH). 1989. Threshold Limit Values and
Biological Exposure Indices for 1989-1990. Cincinnati, OH: Author. Pp. 22, 25, 32.
American Institute of Architects. Academy of Architecture for Health. Guidelines for Design and
Construction of Hospital and Health Care Facilities, 1996-97. Washington, DC: Author. P.58.
American Society of Anesthesiologists. 1974. Occupational Disease Among Operating Room Personnel: A
National Study. Report of an Ad Hoc Committee on the Effect of Trace Anesthetics on the Health of Operating Room
Personnel. Anesthesiology 41: 321-340.
American Society for Testing and Materials. 1988. Standard Specification for Minimum Performance
and Safety Requirements for Components and Systems of Anesthesia Gas Machines. West Conshohocken, PA: Author.
F1161-88.
__________. 1991. Standard Specification for Anesthetic Equipment--Scavenging Systems for
Anesthetic Gases.West Conshohocken, PA: Author. F1343-91.
Askrog, V., and Petersen, R. 1970. Forurening af Operationsstuer Med Lurtformige Anaestetika Og
Reontgenbestraaling. Saertryk Nord Med 83: 501-504.
Axelsson, G., and Rylander, R. 1982. Exposure to Anaesthetic Gases and Spontaneous Abortion: Response Bias in a Postal Questionnaire Study. Int J Epidemiol 11: 250-256.
Azar, I., and Eisenkraft, J.B. 1993. Waste Asnesthetic Gases Spillage and Scavenging Systems. In: Anesthetic Equipment: Principles and Applications, Ehrenwerth, J., and Eisenkraft, J.B., editors, St. Louis, MO, Mosby-Yearbook, Inc. Pp 114-139.
Baden, J.M., and Simmon, V.F. 1980. Mutagenic Effects of Inhalation Anesthetics. Mutat Res 75: 169-189.
Basford, A.B., and Fink, B.R. 1968. The Teratogenicity of Halothane in the Rat. Anesthesiology 29: 1167-1173.
Bowie, E., and Huffman, L.M. 1985. The Anesthesia Machine: Essentials for Understanding. Madison, WI: Ohmeda, The BOC Group, Inc.
Bruce, D.L.1991. Recantation Revisited. Anesthesiology 74: 1160-1161.
Bruce, D.L., Bach, M.J., and Arbit, J. 1974. Trace Anesthetic Effects on Perceptual, Cognitive, and Motor Skills. Anesthesiology 40: 453-458.
Bruce, D.L., and Bach, M.J. 1975. Psychological Studies of Human Performance as Affected by Traces of Enflurane and Nitrous Oxide. Anesthesiology 42: 194-196.
Bruce, D.L., and Stanley, T.H. 1983. Research Replication May Be Subject Specific. Anesth Analg 62: 617.
Buring, J.E., Hennekens, C.H., Mayrent, S.L., Rosner, B., Greenberg, E.R., and Colton, T. 1985. Health Experiences of Operating Room Personnel. Anesthesiology 62: 325-330.
Burkhart, J.E., and Stobbe, T.J. 1990. Real-Time Measurement and Control of Waste Anesthetic Gases During Veterinary Surgeries. Am Ind Hyg Assoc J 51: 640-645.
Cohen, E.N., Bellville, J.W., and Brown, B.W., Jr. 1971. Anesthesia, Pregnancy and Miscarriage. A Study of Operating Room Nurses and Anesthetists. Anesthesiology 35: 343-347.
Cohen, E.N., Brown, B.W., Wu, M.L., Whitcher, C.E., Brodsky, J.B., Gift, H.C., Greenfield, W., Jones, T.W., and Driscoll, E.J. 1980. Occupational Disease in Dentistry and Chronic Exposure to Trace Anesthetic Gases. J Am Dent Assoc 101: 21-31.
Corbett, T.H., Cornell, R.G., Endres, J.L., and Millard, R.I. 1973. Effects of Low Concentrations of Nitrous Oxide on Rat Pregnancy. Anesthesiology 39: 299-301.
Corbett, T.H., Cornell, R.G., Endres, J.L., and Lieding, K. 1974. Birth Defects Among Children of Nurse Anesthetists. Anesthesiology 41: 341-344.
Dorsch, J.A., and Dorsch, S.E. 1984. Understanding Anesthesia Equipment. Second Edition. Baltimore, MD: Williams and Wilkins. Pp. 247-279.
Dorsch, J.A., and Dorsch, S.E. 1994. Understanding Anesthesia Equipment: Construction, Care and Complications. Third Edition. Baltimore, MD: Williams and Wilkins. P.67.
Eichhorn, J.H. 1993. Anesthesia Equipment:Checkout and Quality Assurrance. In: Anesthesia Equipment: Principles and Applications. Ehrenwerth, J., and Eisenkraft, J.B., editors, St. Louis, MO, Mosby-Yearbook, Inc. Pp. 473-511.
Emergency Care Research Institute. 1991. Technology for Anesthesia. 12, No. 4-5:2.
Fink, B.R., Shepard, T.H., and Blandau, R.J. 1967. Teratogenic Activity of Nitrous Oxide. Nature 214: 146-148.
Gandolfi, A.J., White, R.D., Sipes, I.G., and Pohl, L.R. 1980. Bioactivation and Covalent Binding of Halothane in Vitro: Studies with [3H]- and [14C] Halothane. J Pharmacol Exp Ther 214: 721-725.
Garro, A.J., and Phillips, R.A. 1978. Mutagenicity of the Halogenated Olefin, 2- Bromo- 2 - Chloro - 1,1-difluoroethylene, a Presumed Metabolite of the Inhalation Anesthetic Halothane. Mutat Res 54: 17-22.
Guirguis, S.S., Pelmear, P.L., Roy, M.L., and Wong, L. 1990. Health Effects Associated with Exposure to Anaesthetic Gases in Ontario Hospital Personnel. Br J Ind Med 47: 490-497.
Hallen, B., Ehrner-Samuel, H., and Thomason, M. 1970. Measurements of Halothane in the Atmosphere of an Operating Theatre and in Expired Air and Blood of the Personnel During Routine Anaesthetic Work. Acta Anaesth Scand 14: 17-27.
Henry, R.J., and Jerrell, R.G. 1990. Ambient Nitrous Oxide Levels During Pediatric Sedations. Pediatr Dent 12: 87-91.
Holmes, M.A., Weiskopf, R.B., Eger, E.I., Johnson, B.H., and Rampil, I.J. 1990. Hepatocellular Integrity in Swine After Prolonged Desflurane (I-653) and Isoflurane Anesthesia: Evaluation of Plasma Alanine Aminotransferase Activity. Anesth and Analg 71: 249-253.
Huffman, L.M. 1991. Common problems in waste gas management. AANA 59: 109-112.
Jastak, J.T. 1989. Nitrous Oxide in Dental Practice. Int Anesthesiol Clin 27: 92-97.
Kestenberg, S.H., and Young, E.R. 1988. Potential Problems Associated with Occupational Exposure to Nitrous Oxide. J Can Dent Assoc 54: 277-286.
Knill-Jones, R.P., Rodrigues, L.V., Moir, D.D., and Spence, A.A. 1972. Anaesthetic Practice and Pregnancy: Controlled Survey of Women Anaesthetists in the United Kingdom. Lancet 1: 1326-1328.
Knill-Jones, R.P., Newman, B.J., and Spence, A.A. 1975. Anaesthetic Practice and Pregnancy: Controlled Survey of Male Anaesthetists in the United Kingdom. Lancet 2: 807-809.
Lauwerys, R., Siddons, H., Misson, C.B., et al. 1981. Anaesthetic Health Hazards Among Belgian Nurses and Physicians. Int Arch Occup Environ Health 48: 195-203.
McGlothlin, J.D., Jensen, P.A., Fischbach, T.J., Hughes, R.T., and Jones, J.H. 1992. Control of Anesthetic Gases in Dental Operatories. Scand J Work Environ Health 18 Suppl 2:103-105.
National Institute for Occupational Safety and Health. 1977. Criteria for a recommended standard: Occupational Exposure to Waste Anesthetic Gases and Vapors. Cincinnati, OH: U.S.Department of Health, Education, and Welfare. Public Health Service. Center for Disease Control. National Institute for Occupational Safety and Health. DHEW (NIOSH) Publication No. 77-140.
__________. 1994. Control of Nitrous Oxide in Dental Operatories. Cincinnati, OH: U.S.Department of Health and Human Services. Public Health Service. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health. DHHS (NIOSH) Publication No. 94-129.
Pharoah, P.O.D., Alberman, E., Doyle, P., and Chamberlain, G. 1977. Outcome of Pregnancy Among Women in Anaesthetic Practice. Lancet 1: 34-36.
Popova, S., Virgieva, T., Atanasova, J., Atanasov, A., and Sahatchiev, B. 1979. Embryotoxicity and fertility Study with Halothane Subanesthetic Concentration in Rats. Acta Anaesth Scand. 23: 505-512.
Purdham , J.T. 1986. Anesthetic Gases and Vapors (p86-21E). Hamilton, ON: Canadian Centre for Occupational Health and Safety.
Rosenberg, P., and Kirves, A. 1973. Miscarriages Among Operating Theatre Staff. Acta Anaesth Scand Suppl 53: 37-42.
Rosenberg, P.H., and Vanttinnen, H. 1978. Occupational Hazards to Reproduction and Health in Anaesthetists and Paediatricians. Acta Anaesth Scand 22: 202-207.
Rowland, A.S., Baird, D.D., Weinberg, C.R., Shore, D.L., Shy, C.M., and Wilcox, A.J. 1992. Reduced Fertility Among Women Employed as Dental Assistants exposed to High Levels of Nitrous Oxide. N Engl J Med 327: 993-997.
Rowland, A.S., Baird, D.D., Shore, D.L., Weinberg, C.R., Savitz, D.A., and Wilcox, A.J. 1995. Nitrous Oxide and Spontaneous Abortion in Female Dental Assistants. Am J Epidemiol 141: 531-38.
Smith, B.E., Gaub, M.L., and Moya, F. 1965. Teratogenic Effects of Anesthetic Agents: Nitrous Oxide. Anesth Analg 44: 726-732.
Smith, G., and Shirley, A.W. 1978. A Review of the Effects of Trace Concentrations of Anaesthetics on Performance. Br J Anaesth 50: 701-712.
Sweeney, B., Bingham, R.M., Amos, R.J., Petty, A.C., and Cole, P.V. 1985. Toxicity of Bone Marrow in Dentists Exposed to Nitrous Oxide. Br Med J (Clin Res Ed) 291: 567-569.
Tannenbaum, T.N., and Goldberg, R.J. 1985. Exposure to Anesthetic Gases and Reproductive Outcome. J Occ Med 27: 659-668.
Tomlin, P.J. 1979. Health Problems of Anaesthetists and Their Families in the West Midlands. Br Med J 1: 779-784.
United States Department of Labor, Occupational Safety and Health Administration. 1982. Record-keeping and Reporting Occupational Injuries and Illnesses. 29 CFR 1904. Washington, DC: United States Government Printing Office.
__________. 1990. Access to Employee and Medical Records Standard. 29 CFR 1910.1020. Washington, DC: United States Government Printing Office.
__________. 1994. Hazard Communication Standard. 29 CFR 1910.1200. Washington, DC: United States Government Printing Office.
__________. 1991. Chemical Information Manual. Washington, DC: United States Government Printing Office.
United States Food and Drug Administration, HHS. 1994. Anesthesia Apparatus Checkout Recommendations, 1993; Availability. Federal Register, 59(131): 35373-35374.
Vaisman, A.I. 1967. Working Conditions in Surgery and Their Effect on the Health of Anesthesiologists. Eksp Khir Anesteziol 3: 44-49.
Viera, E., Kleaton-Jones, P., Austin, J.C., Moyes, D.G., and Shaw, R. 1980. Effects of Low Concentrations of Nitrous Oxide on Rat Fetuses. Anesth Analg 59: 175-177.
Wharton, R.S., Wilson, A.I., Mazze, R.I., Baden, J.M., and Rice, S.A. 1979. Fetal Morphology in Mice Exposed to Halothane. Anesthesiology. 51:532-537.
Weiskopf, R.B., Eger, E.I., 2d, Ionescu, P., Yasuda, N., Cahalan, M.K., Freire, B., Peterson, N., Lockhart, S.H., Ampil, I.J., and Laster, M. 1992. Desflurane Does Not Produce Hepatic or Renal Injury in Human Volunteers. Anesth and Analg 74: 570-574.
Appendix 1. Glossary.
American Conference of Governmental Industrial Hygienists (ACGIH) is an organization devoted to the development
of administrative and technical aspects of worker health protection. The ACGIH is a professional organization, not a
government agency.
ACGIH threshold limit value-time-weighted average (TLV-TWA) refers to the time-weighted
average airborne concentration of a substance, for a normal 8-hour workday and a 40-hour workweek, to which
nearly all workers may be repeatedly exposed, day after day, without adverse effect.
Adapters are fittings used to establish functional continuity between otherwise disparate and incompatible
components.
Adjustable Pressure-Limiting (APL) Valve, also known as a "pop-off" valve, is
a user-adjustable valve that releases gases to the atmosphere or a scavenging system and is intended to provide control
of the pressure in the breathing system. The volume of gas above that needed to achieve the required patient pressure is vented.
Air is the elastic, invisible mixture of gases (chiefly nitrogen and oxygen) that may be used with
medical equipment; also called medical air.
Anesthesia machine is equipment intended for dispensing and delivering anesthetic gases and vapors into
a breathing system.
Anesthesia system is any of a variety of assemblies designed to administer an anesthetic.
Anesthetic agent is a drug that is used to reduce or abolish the sensation of pain, e.g., halothane,
enflurane, isoflurane, desflurane, sevoflurane, and methoxyflurane.
Anesthetic agent vapor is the gaseous phase of an anesthetic agent that is normally a liquid at room
temperature and atmospheric pressure.
Anesthetic gas is any gaseous substance, e.g., nitrous oxide, used in producing a state of anesthesia.
Anesthetic vaporizer is a device designed to facilitate the change of an anesthetic from a liquid to
a vapor.
Anesthetizing location is any area in a facility where an anesthetic agent or drug is administered in the
course of examination or treatment. This includes operating rooms, delivery rooms, emergency rooms, induction rooms, and other areas.
Area sample is a sample collected at a fixed point in the workplace. The data from the area sample may
or may not correlate with an individual’s personal sample results due to the often high degree of variability in exposures.
Breathing system is a gas pathway in direct connection with the patient's lungs, through which gas flow
occurs at respiratory pressures, and into which a gas mixture of controlled composition may be dispensed. The function of the breathing
system is to convey oxygen and anesthetic gases to the patient's lungs and remove waste and anesthetic gases from the patient's lungs.
Scavenging equipment is not considered part of the breathing system. The system is also referred to as breathing or patient circuit,
respiratory circuit or system.
Breathing system, semiclosed is a system that allows some of the expired gases to leave the circuit; the
remainder mixes with the fresh gases and is reinhaled. A CO2 absorber is used in this system.
Breathing tubes are large-bore, nonrigid tubes composed of rubber or plastic and used in most
breathing systems to convey gases to and from the patient's airway. They are usually corrugated to prevent obstruction due to kinking
and increase flexibility.
Breathing zone is defined as the area immediately adjacent to the employee’s nose and mouth; a hemisphere
forward of the worker’s shoulders with a radius of approximately 6 to 9 inches.
Calibrated vaporizer is an instrument designed to facilitate the change of a liquid anesthetic into its vapor
and to add a controlled amount of this vapor to the fresh gas flow.
Carbon dioxide (CO2) is a colorless, odorless gas, and is a normal end product of human metabolism.
It is formed in the tissues and eliminated by the lungs.
Carbon dioxide absorber is a device used to remove CO2 chemically from exhaled patient gas.
Primarily used in the closed or semiclosed circle breathing system, which requires carbon dioxide absorption to make reinhalation of
previously exhaled gas possible.
Carcinogenicity is the ability of a substance to cause cancer.
Check valves are also known as unidirectional valves, one-way valves, and inspiratory and
expiratory valves (refer to definition of unidirectional valve).
Common (fresh) gas outlet is the port through which the mixture of gases and vapors dispensed from the
anesthesia machine is delivered to the breathing system. Also referred to as the machine outlet.
Compressed gas is defined as any material or mixture having in the container an absolute pressure exceeding
40 psig at 70°F or having an absolute pressure exceeding 104 psig at 130°F.
Congenital anomaly is a structural or functional abnormality of the human body that develops before birth but
is not inherited. One type of birth defect.
Connectors are fittings intended to join together two or more components.
Cylinder supply source is a cylindrical-shaped tank that is color-coded and
pin-indexed or Compressed Gas Association (CGA) valve-specific and used to contain a specified medical gas. It
supplies compressed gas to the anesthesia machine if a pipeline supply source is not available or if the pipeline fails. Cylinders range
in size from B (smallest) to H (largest).
Cylinder pressure gauge monitors the pressure of gas within a cylinder.
Diameter Index Safety System (DISS) provides threaded noninterchangeable (gas-specific) connections
for medical gas lines at pressures of 200 psig or less to minimize the risk of misconnection.
Embryolethal refers to a substance that is lethal to the developing embryo, the product of conception up to
the end of the eighth week of human pregnancy.
Epidemiology is the study of health and illness in human populations. It is the study of trends and events in
similar populations, for example, one exposed to a chemical and one not exposed.
Excess gases are those gases and anesthetic vapors that are delivered to the breathing circuit in excess of
the patient’s requirements and the breathing circuit’s capacity. These gases are released from the breathing circuit via the APL or
pop-off valve or the ventilator pressure relief valve and are ultimately removed from the breathing circuit by the waste
gas scavenging system.
Exhalation check valve, also known as expiratory unidirectional valve, refers to that valve placed in the
vicinity of the CO2 absorber that ensures that exhaled gases flow away from the patient and into the absorber.
Flow control valve, also known as the needle valve, controls the rate of flow of a gas through its associated
flow meter by manual adjustment of a variable orifice.
Flowmeter is a device that measures and indicates the flow rate of a gas passing through it.
Gas is defined as a formless fluid that expands readily to fill any containing vessel, and which can be
changed to the liquid or solid state only by the combined effect of increased pressure and decreased temperature.
Gas-tight seal is a connection that does not allow bubbling when immersed in water and subjected to a
differential pressure.
General anesthesia is a state of unconsciousness in which there is an absence of pain sensation.
Hanger yoke is a device used to attach a reserve gas cylinder to the anesthesia machine. The functions
of the hanger yoke are to orient and support the cylinder, provide a gas-tight seal, and ensure a unidirectional flow
of gas into the machine. It is pin-indexed according to a gas-specific safety system in order to prevent
the connection of a cylinder of one gas to a yoke intended for
another.
HVAC system, also known as the heating, ventilating, and air conditioning system, supplies outdoor replacement
(make-up) air and environmental control to a space or building. It conditions the air by supplying the required degree of air
cleanliness, temperature and/or humidity.
Inhalation check valve, also called inspiratory unidirectional valve, refers to the valve placed in the
vicinity of the CO2 absorber that ensures that the gases flow toward the patient.
In vitro describes studies that are done in the laboratory, literally"in glass," using, for example,
cells, as distinct from studies performed using whole living animals.
Medical gas is any gaseous substance that meets medical purity standards and has application in a medical
environment. Examples are oxygen, nitrous oxide, helium, air, nitrogen, and carbon dioxide.
Medical gas mixture is a mixture of two or more medical gases to be used for a specific medical application.
Mutagenicity is the ability of a substance to cause changes in the genetic material.
NIOSH RELs (recommended exposure limits) are occupational exposure limits recommended by NIOSH as being
protective of worker health and safety over a working lifetime. These limits are generally expressed as 8- or
10-hour TWAs for a 40-hour workweek. The REL may also be expressed as a short-term (TWA)
exposure limit or a ceiling limit.
Nitrous oxide (N2O) is used as an anesthetic agent in medical, dental, and veterinary operatories.
It is a weak anesthetic with rapid onset and rapid emergence. In hospitals, it may be used with oxygen as a carrier gas for other,
more potent anesthetics. In dental offices, it is administered with oxygen, primarily as an analgesic (an agent that diminishes or
eliminates pain in the conscious patient) and as a sedative to reduce anxiety.
Nonrecirculating ventilation system takes in fresh outside air and processes it by filtering and adjusting
the humidity and temperature. The processed air is circulated through the various rooms in a facility, and then all of it is exhausted
to the atmosphere. Whatever volume of fresh air is introduced into a room is ultimately exhausted outdoors.
Occupational exposure to waste anesthetic gases includes exposure to any inhalation anesthetic agents that
escape into locations associated with, and adjacent to, anesthetic procedures. Such locations include, but are not limited to,
operating rooms, delivery rooms, recovery rooms, and dental operatories.
Oxygen (O2) is an element which, at atmospheric temperatures and pressures, exists as a colorless,
odorless, tasteless gas. Its utstanding properties are its ability to sustain life and to support combustion. Although oxygen is nonflammable, materials which burn in air will burn much more vigorously and create higher temperatures in oxygen or oxygen-enriched atmospheres.
Oxygen flush valve is a separate valve designed to rapidly supply a large volume of oxygen to the
breathing system.
PACU (postanesthesia care unit) is also known as the recovery room.
Patient end is the end of the component part nearest the patient.
PEEP valve is a device installed in the exhalation limb of the patient circuit that allows positive
end-expiratory pressure to be delivered to the patient's airway and adjusted as needed.
Personal sample is a sample collected from an individual’s breathing zone.
Pin Index Safety System is a safeguard to eliminate cylinder interchanging and the possibility of accidentally
placing the incorrect gas on a yoke designed to accommodate another gas. Two pins on the yoke are so arranged that they project into
the cylinder valve. Each gas or combination of gases has a specific pin arrangement.
Pipeline supply source is a permanently installed piped distribution system that delivers medical gases such
as oxygen, nitrous oxide, and air to the operating room.
Pneumatic means pertaining to or operated by air or other gas under pressure.
Power outlet is an accessory outlet located on an anesthesia machine that supplies a driving gas for auxiliary
equipment such as a ventilator. Driving gas is normally oxygen, but medical air may be used.
Pressure relief valve is a mechanical device that eliminates system overpressure by allowing the controlled
or emergency escape of liquid or gas from a pressurized system. The relief valve may or may not be adjustable.
Prospective study or cohort study follows a population from a set time into the future. It is an
epidemiological method for identifying the future relationship, if any, between exposure to an agent and the increased incidence
of some adverse health effect in a population.
PSIG stands for pounds per square inch gauge, which is the difference between the measured pressure
and surrounding atmospheric pressure. Most gauges are constructed to read 0 at atmospheric pressure.
Recirculating ventilation system returns part of the exhaust air to the air supply duct. The system takes
in an amount of fresh outside air that varies as a function of the outside temperature. Air exhausted from a room is filtered for
particulate matter and bacteria, not anesthetic gases, and then recirculated through several rooms by means of a common mixing (plenum)
chamber. In this process, some fresh air is added and a equal amount of recirculating air is exhausted.
Recovery room is the patient care location where recovering patients are awakened and stabilized and/or
awakened after surgical anesthesia. Anesthetic gases are exhaled by recovering patients (who received inhalation anesthetics) as
they breathe.
Reservoir bag is also known as the respiratory bag or breathing bag. It allows accumulation of gas during
exhalation so that a reservoir is available for the next inspiration. It provides a means whereby anesthesia personnel may assist or
control ventilation. It can serve, through visual and tactile observation, as a monitor of a patient’s spontaneous respirations and
acts to protect the patient from excessive pressure in the breathing
system.
Respiration is the process by which a rapid exchange of oxygen and carbon dioxide takes place between the
atmosphere and the blood coming to the pulmonary capillaries. Oxygen is taken up, utilized in metabolic processes, and a proportional
amount of carbon dioxide is released.
Retrospective study or case control study examines two populations. The first population consists of
individuals who demonstrate the effect of interest, and the second is made up of those who do not. The two populations are matched as
well as possible with respect to all other variables, e.g., age, socioeconomic status, and so on. Then the past histories of exposure
of the two populations are investigated to determine if some differences can be identified that might be related to the toxic effects
observed.
Scavenging is defined as the collection of excess gases from the breathing circuit and removal of these
gases to an appropriate place of discharge outside the working environment.
Scavenging system is defined as a device (assembly of specific components) that collects and removes the
excess anesthetic gases that are released from the breathing circuit. Scavenging systems are also called evacuation systems, waste
anesthetic gas disposal systems, and excess anesthetic gas-scavenging systems.
Source-control technology is an engineering control designed to collect and remove excess anesthetic gases
at the point of origin (i.e., from the breathing circuit or in close proximity to the patient’s mouth and nose). It can be either a
scavenging system or local (auxiliary) exhaust ventilation system.
Source sample is a sample collected at the origin of contamination (source of emission).
Teratogenicity is the ability of a substance to cause birth defects in offspring, as a result of maternal
(before or after conception) or paternal exposure to the toxic substance.
Tracheal tube also called the endotracheal tube, intratracheal tube, and catheter is inserted into the trachea
and is used to conduct gases and vapors to and from the lungs.
TWA is a time-weighted average concentration. It is a way of expressing exposure such that the
amount of time spent exposed to each different concentration level is weighted by the amount of time the worker was exposed to that
level.
Unidirectional valve is a valve that allows gas flow in one direction only. Two unidirectional valves are
used in each circle system to ensure that the gases flow toward the patient in one limb of the circle breathing system and away in
the other. They are usually part of the absorber assembly.
Vapor is the gaseous phase of a substance which at ordinary temperature and pressure exists as a liquid.
Ventilation is (1) the physical process of moving gases into and out of the lungs. (2) It is also defined
for the purposes of industrial hygiene engineering as a method for providing control of an environment by strategic use of airflow.
The flow of air may be used to provide either heating or cooling of a work space, to remove a contaminant near its source of release
into the environment, to dilute the concentration of a contaminant to acceptable levels, or to replace air exhausted from a space.
Waste anesthetic gases are those gases that are inadvertently released into the workplace and/or can no
longer be used. They include all fugitive anesthetic gases and vapors that are released into anesthetizing and recovery locations,
from equipment used in administering anesthetics under normal operating conditions, as well as those gases that leak from the anesthetic
gas scavenging system, or are exhaled by the patient into the workplace environment. Waste gases are also those excess gases in the
breathing circuit that are ultimately scavenged. Spills of liquid anesthetic agents also contribute to ambient levels of waste gases.
Waste anesthetic gases may include N2O and vapors of potent inhaled volatile anesthetic agents such as halothane,
enflurane, isoflurane, desflurane and sevoflurane.
Appendix 2. Food and Drug Administration (FDA) Anesthesia Apparatus Checkout Recommendations, 1993.
This checkout, or a reasonable equivalent, should be conducted before administration of anesthesia. These recommendations are only valid
for an anesthesia system that conforms to current and relevant standards and includes an ascending bellows ventilator and at least the
following monitors: capnograph, pulse oximeter, oxygen analyzer, respiratory volume monitor (spirometer) and breathing system pressure
monitor with high and low pressure alarms. This is a guideline which users are encouraged to modify to accommodate differences in
equipment design and variations in local clinical practice. Such local modifications should have appropriate peer review. Users should
refer to the operator’s manual for the manufacturer’s specific procedures and precautions, especially the manufacturer’s low pressure
leak test (step #5).
Note: *If an anesthesia provider uses the same machine in successive cases, these steps need not be repeated
or may be abbreviated after the initial checkout.
Emergency Ventilation Equipment
|
*1. Verify Backup Ventilation Equipment is Available & Functioning
|
High-Pressure System
|
*2. Check Oxygen Cylinder Supply
- Open O2 cylinder and verify at least half full (about 1000 psi).
- Close cylinder.
*3. Check Central Pipeline Supplies
- Check that hoses are connected and pipeline gauges read about 50 psi.
|
Low-Pressure System
|
*4. Check Initial Status of Low-Pressure System
- Close flow control valves and turn vaporizers off.
- Check fill level and tighten vaporizer’s filler caps.
*5. Perform Leak Check of Machine Low-Pressure System
- Verify that the machine master switch and flow control valves are OFF.
- Attach"Suction Bulb" to common (fresh) gas outlet.
- Squeeze bulb repeatedly until fully collapsed.
- Verify bulb stays fully collapsed for at least 10 seconds.
- Open one vaporizer at a time and repeat"c" and"d" as above.
- Remove suction bulb, and reconnect fresh gas hose.
* 6. Turn On Machine Master Switch and all other necessary equipment.
* 7. Test Flowmeters
- Adjust flow of all gases through their full range, checking for smooth operation of floats and undamaged flowtubes.
- Attempt to create a hypoxic O2/N2O mixture and verify correct changes in flow and/or alarm.
|
Scavenging System
|
* 8. Adjust and Check Scavenging System
- Ensure proper connections between the scavenging system and both APL (pop-off) valve and ventilator relief valve.
- Adjust waste gas vacuum (if possible).
- Fully open APL valve and occlude Y-piece.
- With minimum O2 flow, allow scavenger reservoir bag to collapse completely and verify that absorber pressure
gauge reads about zero.
- With the O2 flush activated, allow the scavenger reservoir bag to
distend fully, and then verify that absorber pressure gauge reads <10 cm H2O.
|
Breathing System
|
* 9. Calibrate O2 Monitor
- Ensure monitor reads 21% in room air.
- Verify low O2 alarm is enabled and functioning.
- Reinstall sensor in circuit and flush breathing system with O2.
- Verify that monitor now reads greater than 90%.
10. Check Initial Status of Breathing System
- Set selector switch to "Bag" mode.
- Check that breathing circuit is complete, undamaged and unobstructed.
- Verify that CO2 absorbent is adequate.
- Install breathing circuit accessory equipment (e.g., humidifier, PEEP valve) to be used during the case.
11. Perform Leak Check of the Breathing System.
- Set all gas flows to zero (or minimum).
- Close APL (pop-off) valve and occlude Y-piece.
- Pressurize breathing system to about 30 cm H2O with O2 flush.
- Ensure that pressure remains fixed for at least 10 seconds.
- Open APL (pop-off) valve and ensure that pressure decreases.
|
Manual and Automatic Ventilation Systems
|
12. Test Ventilation Systems and Unidirectional Valves
- Place a second breathing bag on Y-piece.
- Set appropriate ventilator parameters for next patient.
- Switch to automatic ventilation (Ventilator) mode.
- Fill bellows and breathing bag with O2 flush and then turn ventilator ON.
- Set O2 flow to minimum, other gas flows to zero.
- Verify that during inspiration bellows delivers appropriate tidal volume and that during expiration bellows fills completely.
- Set fresh gas flow to about 5 L/min.
- Verify that the ventilator bellows and simulated lungs fill and empty appropriately without
sustained pressure at end expiration.
- Check for proper action of unidirectional valves.
- Exercise breathing circuit accessories to ensure proper function.
- Turn ventilator OFF and switch to manual ventilation (Bag/APL) mode.
- Ventilate manually and assure inflation and deflation of artificial lungs and appropriate feel of system resistance and compliance.
- Remove second breathing bag from Y-piece.
|
Monitors
|
13. Check, Calibrate and/or Set Alarm Limits of all Monitors
Capnometer
Oxygen Analyzer
Pressure Monitor with High and Low Airway Alarms
Pulse Oximeter
Respiratory Volume Monitor (Spirometer)
|
Final Position
|
14. Check Final Status of Machine
- Vaporizers off
- APL valve open
- Selector switch to "Bag"
- All flowmeters to zero
- Patient suction level adequate
- Breathing system ready to use
|
Appendix 3. Scavenging System, Interface Component
The interface serves to prevent potentially dangerous increases or decreases of pressure in the anesthetic waste gas
disposal system from reaching the patient’s breathing circuit. In order to do this, the interface has three components: positive pressure
relief, negative pressure relief, and a reservoir.
Irrespective of the type of disposal system used (i.e., active or passive), positive pressure relief must be provided to protect the
equipment and patient if occlusion of the scavenging system outlet occurs. If the scavenging system outlet becomes occluded,
the positive-pressure relief vent opens to prevent transmission of high pressure to the breathing circuit. If an active
disposal system is used, negative pressure relief is needed to prevent negative (suction) pressure from the disposal system from
reaching the patient’s breathing circuit. A reservoir is necessary to allow the scavenging system to accommodate an increased
volume of excess anesthetic gas which may transiently exceed the per-minute removal capacity of the system. It may
also serve as a monitor of the scavenging system if the reservoir is a distensible bag. Overdistension of the bag could indicate
inadequate function of the system and the need to adjust the needle valve to allow more gas to flow through.
Interfaces can be divided into two types: open and closed, depending on the means to provide positive and negative pressure relief. An
open reservoir interface is one that is always open to atmosphere and contains no valves. It relies on open ports for positive and
negative pressure relief. A closed interface uses "spring-loaded or weighted" valves for positive and negative
pressure relief.
The open reservoir interface (Figure 9) should be used only with an active disposal system. Because the discharge of waste gases from the
breathing system is usually intermittent and flow through an active disposal assembly is continuous, a reservoir is needed to accommodate
the surges of gas that enter the interface at a flow rate greater than that at which the disposal system removes them. The reservoir
allows the flow rate in the disposal system to be kept just above the average, rather than at the peak flow rate of gases from
the gas-collecting assembly.
A closed interface is one in which the connection(s) with the atmosphere is(are) through valve(s). A positive pressure relief is always
required to allow release of gases into the room if there is an obstruction of the scavenging system downstream of the interface.
If an active disposal system is to be used, a negative pressure relief valve is necessary to allow entrainment of room air when the
pressure falls below atmospheric.
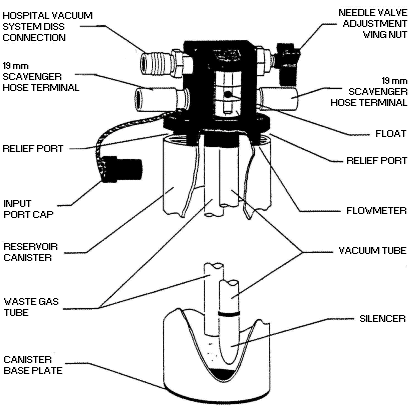
Figure 9
Open reservoir scavenging interface.
(Reproduced by permission of North American Dräger, Telford, Pennsylvania).
|
The interface typically consists of a manifold with four ports and two relief
valves (Azar and Eisenkraft 1993;
Dorsch and Dorsch 1994).
Figure 10 shows the flow of waste gases from the breathing circuit as it enters the intake ports
of the interface. This figure shows the pathway of gas flow in an active scavenging system that uses a
facility’s vacuum source (wall suction) for gas disposal (Huffman 1991).
As gas is drawn through the suction nipple, located on the right of the drawing in Figure 10,
it flows through the manifold and past the two relief valves. The upper relief valve limits positive pressure, and the lower valve limits
negative pressure. A 3-liter bag is shown attached in the diagram and serves as the waste gas reservoir. When more flow is
passing into the manifold than the vacuum can remove, waste gas is temporarily stored in the reservoir bag.
The rate of gas flow through the interface is controlled by adjusting the needle valve in such a way that the reservoir bag is not allowed
to become filled. In the ideal situation, this rate of flow should maintain the volume in the reservoir bag between empty and
half-filled. Adjusting the needle valve alters the flow of waste gases into the vacuum source. This adjustment does not
regulate vacuum or suction. If the flow is insufficient and the reservoir bag is allowed to distend, the positive pressure relief valve
will open and vent some of the exhaled gases into the room. This situation is corrected simply by adjusting the needle valve to increase
the flow of waste gases to the vacuum. If the flow is too great and the bag collapses, the negative pressure relief valve will open and
let in as much room air as needed.
The purpose of these valves is to protect the breathing circuit from extremes of pressure. The positive pressure relief valve will not be
activated if the flow is properly adjusted and the contour of the bag is observed to monitor its volume. In an active scavenging system, any
unused nipple must be capped or the vacuum will draw in room air and also provide the opportunity for waste gases to diffuse into the room.
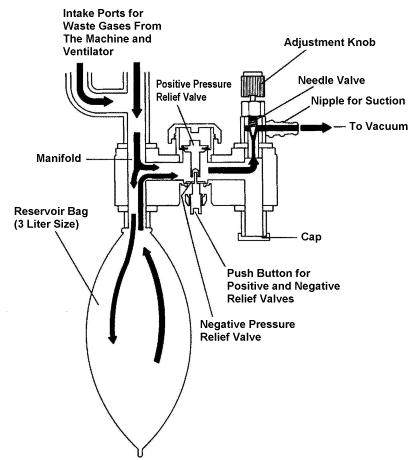
Figure 10
The flow of waste gases through the scavenging interface that is connected to a vacuum source.
(Reproduced by permission of Datex·Ohmeda, Madison, Wisconsin). |
A passive scavenging system for waste gas evacuation, shown in
Figure 11, uses the facility’s ventilation system instead of the vacuum system to dispose of
waste gases. In this configuration, flow of waste gases through the interface is basically the same as in the active system. Gas pressure
is limited by positive and negative relief valves. Transfer of the waste gases from the interface to the disposal system relies solely on
the pressure of the waste gases since a vacuum is not used.
In a passive system the adjustment knob must remain in the down position to close the needle valve. As shown below, a 19 mm corrugated hose
is used to connect the interface with the room’s ventilation exhaust grille
(Azar and Eisenkraft 1993). A passive system (unlike an active
system) is not connected to a vacuum or source of negative pressure and does not need to be adjusted regularly.
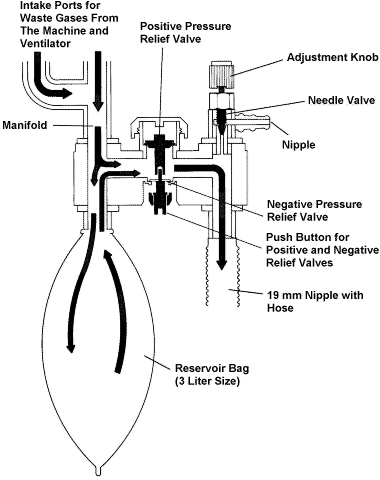
Figure 11
The flow of waste gases through the interface in a passive scavenging system.
(Reproduced by permission of Datex·Ohmeda, Madison, Wisconsin). |
|
|

|
| |

|

