Contact Us
- 800–CDC–INFO
- (800-232-4636)
- 888–232–6348 (TTY)
- cdcinfo@cdc.gov
2008-09 INFLUENZA PREVENTION & CONTROL RECOMMENDATIONS
Timing of Vaccination
Vaccination efforts should be structured to ensure the vaccination of as many persons as possible over the course of several months, with emphasis on vaccinating before influenza activity in the community begins. Even if vaccine distribution begins before October, distribution probably will not be completed until December or January. The following recommendations reflect this phased distribution of vaccine.
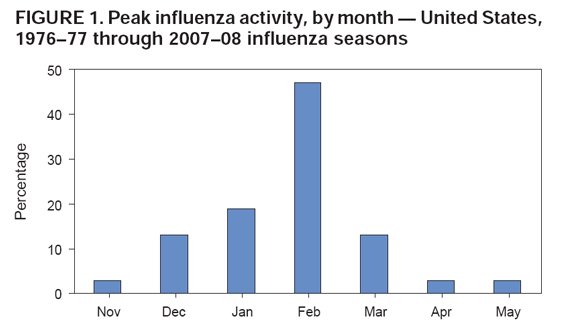
In any given year, the optimal time to vaccinate patients cannot be precisely determined because influenza seasons vary in their timing and duration, and more than one outbreak might occur in a single community in a single year. In the United States, localized outbreaks that indicate the start of seasonal influenza activity can occur as early as October. However, in >80% of influenza seasons since 1976, peak influenza activity (which is often close to the midpoint of influenza activity for the season) has not occurred until January or later, and in >60% of seasons, the peak was in February or later (Figure 1). In general, health-care providers should begin offering vaccination soon after vaccine becomes available and if possible by October. To avoid missed opportunities for vaccination, providers should offer vaccination during routine health-care visits or during hospitalizations whenever vaccine is available.
Vaccination efforts should continue throughout the season, because the duration of the influenza season varies, and influenza might not appear in certain communities until February or March. Providers should offer influenza vaccine routinely, and organized vaccination campaigns should continue throughout the influenza season, including after influenza activity has begun in the community. Vaccine administered in December or later, even if influenza activity has already begun, is likely to be beneficial in the majority of influenza seasons. The majority of adults have antibody protection against influenza virus infection within 2 weeks after vaccination.
All children aged 6 months--8 years who have not received vaccination against influenza previously should receive their first dose as soon after vaccine becomes available as is feasible. This practice increases the opportunity for both doses to be administered before or shortly after the onset of influenza activity.
Persons and institutions planning substantial organized vaccination campaigns (e.g., health departments, occupational health clinics, and community vaccinators) should consider scheduling these events after at least mid-October because the availability of vaccine in any location cannot be ensured consistently in early fall. Scheduling campaigns after mid-October will minimize the need for cancellations because vaccine is unavailable. These vaccination clinics should be scheduled through December, and later if feasible, with attention to settings that serve children aged 6--59 months, pregnant women, other persons aged <50 years at increased risk for influenza-related complications, persons aged 50 years and older, HCP, and persons who are household contacts of children aged 59 months and younger or other persons at high risk. Planners are encouraged to develop the capacity and flexibility to schedule at least one vaccination clinic in December. Guidelines for planning large-scale vaccination clinics are available at http://www.cdc.gov/flu/professionals/vaccination/vax_clinic.htm.
During a vaccine shortage or delay, substantial proportions of TIV doses may not be released and distributed until November and December or later. When the vaccine is substantially delayed or disease activity has not subsided, providers should consider offering vaccination clinics into January and beyond as long as vaccine supplies are available. Campaigns using LAIV also can extend into January and beyond.
NOTE: The text above is taken from Prevention & Control of Influenza - Recommendations of the Advisory Committee on Immunization Practices (ACIP) 2008. MMWR 2008 Jul 17; Early Release:1-60. (Also available as PDF, 586K).
- Page last updated August 29, 2008
- Content Source: Coordinating Center for Infectious Diseases (CCID)
- National Center for Immunization and Respiratory Diseases (NCIRD)