|
|
|
|
|
|
Tips for Worksheet Users
- Be sure to look at the "Air & Water Reactions" information about each substance to find out whether it is air- or water-reactive. To do this, search for the substance of concern [from the Mixture Worksheet, click Add a Chemical, then type in the substance's name or CAS (Chemical Abstracts Service) number]. You'll find the "Air & Water Reactions" section on the card describing the substance (see the example section below).

- Look elsewhere than the Mixture Worksheet to get the full story.
Example: The Worksheet correctly predicts no reaction between lithium aluminum hydride with an ether such as diethylene glycol dimethyl ether (DGD).

But,
- over time, ethers such as DGD can combine with atmospheric oxygen to form peroxides. These peroxides can combine explosively with lithium aluminum hydride.
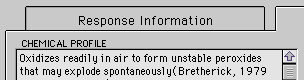
- if the ether contains water, the lithium aluminum hydroxide and water can react?in a potentially violent manner?to form hydrogen.

The Worksheet alerts you to both these hazards:
- Check "Additional Information" about DGD to see that DGD reacts with oxygen to form peroxides.
- Check "Air & Water Reactions" for lithium aluminum hydride to see that it's a water-reactive chemical that can react violently with water to generate hydrogen.
- This version doesn't predict specific reaction products (we hope to add this, at some level, to the next version). It reports things like "flammable gas generation" or "liberates toxic gas." When you see a statement like this in the Mixture Worksheet, check for more information about the reactants; you can sometimes find out what the product would be.
Example: When you combine nitric acid and sodium hydrosulfide, one of the hazard statements is "flammable gas generation."

Check "Air & Water Reactions" to see that sodium hydrosulfide mixed with either water or an acid generates flammable hydrogen sulfide vapors.

- Generally, chemicals in the database are hazardous. The "Special Hazard" denotations are for especially acute hazards. (Examples of Special Hazards are "Air reactive" and "Peroxidizable." But no "Special Hazards" notation doesn't mean no hazards.
- Materials that pose certain special or acute hazards have been assigned to nine hazard classes as well as to reactivity groups. These hazard classes include:
- highly flammable (101)
- explosive (102)
- polymerizable (103)
- strong oxidizing agent (104)
- strong reducing agent (105)
- water-reactive (107)
- air-reactive (108)
- peroxidizable (111)
- radioactive (400)
Hazard Class Numbers (shown in parentheses in the list above) are numbers designating each hazard class. You can view reactivity information about substances in a particular hazard class by using an advanced search, using the hazard class number as your search criterion.
|
|
|
|


