U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
May 2003
Pharmacology and Toxicology
Guidance for Industry
Photosafety Testing
Additional copies are available from:
Office of Training and Communication
Division of Drug Information, HFD-240
Center for Drug Evaluation and Research
Food and Drug Administration
5600 Fishers Lane
Rockville, MD 20857
(Tel) 301-827-4573
http://www.fda.gov/cder/guidance/index.htm
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
May 2003
Pharmacology and Toxicology
Guidance for Industry
Guidance on Photosafety Testing
This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. An alternative approach may be used if such approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.
This guidance is intended to help applicants decide whether they should test for photoirritation and assess the potential of their drug product to enhance UV-associated skin carcinogenesis. The guidance describes a consistent, science-based approach for photosafety evaluation of topically and systemically administered drug products. Basic concepts of photobiology and phototesting are described, along with a process that can be used to make testing decisions or communicate risks.
Use of the principles expressed in this guidance should reduce unnecessary testing while ensuring an appropriate assessment of photosafety. The document does not recommend specific tests but refers to some available testing methods. Sponsors may choose to use some of these tests to evaluate photoirritation, photochemical carcinogenicity potential, or potential to enhance UV-associated skin carcinogenesis. Sponsors also can propose other assays that are scientifically sound. Tests involving biomarkers in the skin of humans receiving the drug product may clarify mechanisms of direct or indirect photoeffects seen in nonclinical studies (see section IV.C., Mechanistically Based and Other Assays).
Photosafety testing (testing for adverse effects of drug products in the presence of light) is only recommended when it is felt that the results of such testing would yield important safety information or would be informative for the consumer and healthcare practitioner.
The glossary at the end of the document defines abbreviations and important terminology used to describe photobiologic concepts. The clinical definition of photosensitivity includes both phototoxicity (photoirritation) and photoallergy. This document uses the clinical definition but addresses nonclinical testing for photochemical irritation (photoirritation) only. At this time, nonclinical models of testing for photoallergy are not considered to be predictive of clinical effects and are not recommended.
Flowcharts for evaluation of photoirritating drug products and nonphotoreactive drug products are also provided at the end of the document. The flowcharts illustrate the decision-making process but do not address all situations that could arise during drug development.
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
Photobiology is the study of the effect of UVA and/or UVB, visible, and IR radiation upon living systems (Smith 1989; Kochevar et al., 1993). The first law of photochemistry (Grotthaus-Draper Law) states that light must be absorbed for a photochemical event to occur (Megaw and Drake 1986). Chromophores in drug products and DNA in dermal tissue are targets for photochemical reactions. Photoirritation and/or photoallergy occur when a photoactive chemical enters the skin by dermal penetration or systemic circulation and becomes excited by appropriate UV or visible light photons.
Fortunately, the skin is an optically heterogeneous medium that modifies the amount of radiation that can reach deeper dermal structures and functions as a protective barrier that minimizes damage from light exposure. Protective mechanisms include reflection, refraction, scattering, and absorption (Kornhauser et al., 1996). Excision-repair and other DNA repair mechanisms of UV-damaged DNA (Hessel et al., 1992; Kraemer et al., 1994; Lindahl et al., 1997) provide further protection against gene mutation and skin cancer.
Photoirritation is a light-induced, nonimmunologic, skin response to a photoreactive chemical. The route of exposure to the photoreactive chemical can be by direct application to the skin or by the circulatory system following systemic administration. Photoirritation reactions resemble primary irritation reactions in that they can be elicited following a single exposure, in contrast to photoallergic reactions, which have an induction period before elicitation of the response. A photoactive chemical can be the parent drug or an excipient in a drug product, or it can be a metabolite, impurity, or degradant. Many diverse classes of drugs (including antimicrobials, NSAIDs, antidepressants, anticonvulsants, diuretics, and antihypertensives) have been reported to cause photoirritation in humans (Holzle et al., 1991; Johnson 1984; Physicians’ Desk Reference 2000). Acute photoirritation reactions can resemble sunburn and may range from a mild erythema to blistered skin with sloughing. Although a relatively small percentage of the population may show clinical symptoms of photoirritation, a much larger percentage may have immediate subclinical effects. Nonclinical tests can identify some photoirritating drug products before widespread clinical exposure occurs, allowing appropriate precautions to be implemented.
Photoallergy is an acquired, immunologically mediated reaction to a chemical activated by light. The occurrence of a photoallergic response to a chemical is idiosyncratic (highly dependent upon the specific immune reactivity of the host). Compounds that elicit a photoirritation response also may be capable of initiating a photoallergic reaction. Examples of photoallergens in humans include promethazine, benzocaine, and
p-aminobenzoic acid (Holzle et al., 1991, Johnson 1984). Photoallergy is best assessed clinically; several approaches for evaluation of clinical photoallergy potential are available.
Data from animals and humans suggest that at least some photoirritants enhance UV-
associated skin carcinogenesis. 8-Methoxypsoralen (8-MOP), used in PUVA therapy (Stern and Lunder 1998), is considered to be a photococarcinogen in humans, while several fluoroquinolones have been demonstrated to be photoirritants and photochemical carcinogens in hairless mice (Physicians’ Desk Reference 2000). However, data for many other classes of pharmaceuticals are unavailable.
Other drug products that are not photochemical irritants can enhance UV-induced skin carcinogenesis. Epidemiologic data (Abel 1989; Frezza et al., 1997; Penn 1988) indicate that persons on chronic immunosuppressive therapy (e.g., cyclosporin following organ transplantation) are at greater risk for skin cancer than the general population. A compound can also enhance UV carcinogenicity indirectly by altering biologic processes or optical or structural features of the skin that function as protective mechanisms. Data from animals exposed to vehicles that decrease protective properties of the skin support this concept (Gibbs et al., 1985).
Changes in the optical properties of the skin, such as those caused by a drug vehicle, can result in a greater UV dose to the viable layers of the skin. Data on correlation of latitude, UV exposure, and cancer risk in humans suggest that an increase in UV exposure as small as 20 percent could result in a 4-fold increase in basal-cell carcinoma (Moan et al., 1999).
Historically, the majority of systemically administered drugs have not undergone controlled testing for determining their potential for photoirritation. Yet a number were later identified as phototoxic to humans. Topically applied dermatologic drugs routinely have been tested for photoirritation in both animals and humans if they absorb light in the UVA, UVB, or visible spectrum. In the absence of data from photoirriation or photoallergy tests conducted in animals or humans, warnings about the potential for photoirritation or photoallergy generally have been added to labels after reports of adverse reactions resulting from widespread clinical use of the products.
Relatively few drug products have been tested to elucidate their potential for enhancing UV-mediated carcinogenic effects on the skin. By itself, UV light is a carcinogen in humans (IARC, 1992). The regulatory issue is whether a drug enhances the carcinogenic effect of UV light to such an extent that it significantly increases the potential human carcinogenic risk, making it important that the patient and the physician be informed. However, testing for photococarcinogenicity in humans is unethical; animal testing has been used as a surrogate. The method that has commonly been used for testing the potential photococarcinogenicity of a compound has been the Skh1-hr hairless mouse model. A positive response in this photococarcinogenicity assay is a decreased time to skin neoplasm development in animals exposed to the test material plus UV radiation (i.e., sunlight simulation), compared with exposure to the same dose of UV radiation alone. Information from this assay has been included in labels and may furnish a frame of reference for comparisons between drugs. Numerous researchers have conducted variants of this assay in several strains of haired mice that had been shaved. However, because of the uncertainties involved in extrapolation from such animal testing to humans and the apparent insensitivity of this assay to some topical immunosuppressants and topical photogenotoxicants, other scientifically valid methods providing relevant information for assessing the long-term adverse photoeffects of drug products on biomarkers in human skin are desirable.
For most drugs, it is generally adequate to test only the drug substance without the excipients for adverse photoeffects. For topical products that will be applied to sun-exposed skin, FDA recommends that the drug product, not just the active ingredient, be evaluated under conditions of simulated sunlight. This is because many excipients in these types of products modify the skin, and dermal applications usually deliver relatively large amounts of both parent drug and vehicle to the skin. Many researchers have reported the effects of topically applied vehicles on the skin, some of which alter the optical properties of human skin. Some examples of these effects are as follows:
- Pharmaceutical vehicles (e.g., creams, gels, lotions, or solutions) can decrease the amount of light reflected, scattered, or absorbed in the skin (Anderson and Parrish 1981; Serup et al., 1989) or increase the percutaneous absorption of drugs in the skin of humans and mice (Marzulli and Maibach 1991; Baynes et al., 1996).
- Vehicles can increase or decrease adverse photoproperties (Kaidbey and Kligman 1974; Dearman et al., 1996) or photostability of drug products (Asker and Harris 1988; Islam and Asker 1995; Marti-Mestres et al., 1997). Vehicles can enhance the effects of other components in the formulation and (1) increase epidermal thickening in rodent skin (Wrench 1980), (2) change collagen gene expression in hairless mice (Chaquor et al., 1997), or (3) influence the solubility and general stability of the drugs (Chellquist and Gorman 1992).
- Some cream-based vehicles have been found to be photosensitizers themselves (proprietary), while some oil-based emollients can increase UVB transmission and UV carcinogenicity in mice (Gibbs et al., 1985).
Nonclinical tests for photochemical irritation are considered predictive of human effects. The intent of the procedures discussed below is to ascertain the potential of pharmaceuticals to elicit a photochemical irritation reaction before widespread human use. The process attempts to address these safety concerns adequately while optimizing the use of resources. To accomplish this goal, a decision tree approach is recommended to assess whether testing should be conducted and what type of testing may be appropriate. Other approaches may also accomplish this goal. It is recognized that even short-term exposure to some nonphotoreactive drugs in the presence of ultraviolet light could result in adverse effects in the skin (e.g., those that can immediately change the optical properties of the skin).
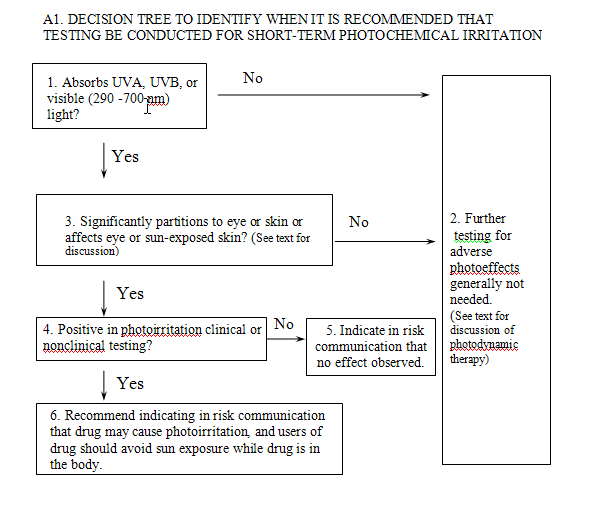
Short-term photoirritation testing in animals, perhaps followed by photoirritation and photoallergy studies in humans, should be considered for all drug substances and formulation components that absorb UVB, UVA, or visible radiation (290-700 nm) and (1) are directly applied to the skin or eyes, or significantly partition to one of these areas when administered systemically, or (2) are known to affect the condition of the skin or eyes (see Flowchart A1). A drug product would not be considered for testing for photoirritation potential if the person receiving the drug would not be exposed to light in the sunlight spectrum while the drug or photoactive metabolites were in the body. Additionally, it would not be appropriate to conduct photochemical irritation testing on a drug product that was applied only to skin not exposed to the sun if the drug did not undergo significant distribution to sun-exposed areas.
A description of the flowchart testing paradigm follows. Information regarding the ultraviolet/visible radiation absorption spectrum for the drug substance or drug formulation, as appropriate, is important in making a testing decision. A spectroscopic scan will determine if a drug absorbs between 290 and 700 nm of the electromagnetic spectrum. The scan is an important component of the safety assessment. Presentation of only absorption maxima will not adequately address safety concerns. Drug products that do not absorb between 290 and 700 nm will not be photoactivated (Box 1). Therefore, they cannot be direct photochemical photosensitizers (Box 2). Some drugs elicit a photosensitivity reaction that is unrelated to the UV absorbance of the administered drug. These secondary mechanisms include perturbation of heme synthesis and increased formation of other light-absorbing endogenous molecules resulting from administration of nonlight-absorbing drugs (e.g., aminolevulinic acid, Physicians’ Desk Reference 2000). These effects may be identified from standard toxicologic testing.
In addition to absorption of UV or visible radiation, the drug (or metabolites) should reach the skin or eye at levels sufficient to cause photoirritation reactions (Boxes 3 and 4). Tissue distribution studies of systemically administered drug products, usually included in IND submissions, can be used to assess the extent of partitioning into the skin or eyes. In the absence of partitioning into light-exposed compartments, photoirritation testing is unlikely to be informative and need not be conducted. However, agents used for photodynamic therapy might be an exception, and valuable safety information (e.g., effects on internal organs after exposure to operating room lighting) can be generated even if partitioning into the skin or eyes does not occur.
When drugs are identified as photoirritants, FDA recommends that the risk communication include a warning to avoid sun exposure (Box 6). In the absence of human data, a drug shown to be a photoirritant in nonclinical studies could be indicated as potentially causing photosensitivity. When adequate human data addressing photoirritation are available, they would be included in the description of the product and would supplant animal data.
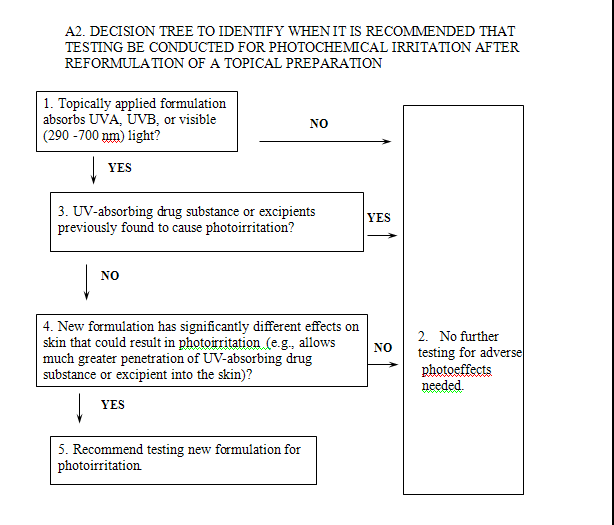
In general, reformulations intended for administration by routes other than topical to the skin do not have to be tested, provided that any new excipients undergo appropriate evaluation. It is also not necessary to test most reformulations of a topical product for nonclinical photoeffects. If the drug substance or excipients have previously been shown to cause photoirritation, additional nonclinical photoirritation testing is generally not needed (Box 3). However, FDA recommends that excipient changes that could modify adverse photoeffects on the skin be tested (Boxes 4 and 5). For example, the Agency recommends that a switch to a cream formulation from an ethanolic solution generally be evaluated for photoeffects. Information on the photoirritant properties of excipients and their effects on the penetration of the drug substance into the skin would be useful in further defining whether new formulations should be studied. Studies of dermal absorption of the drug substance for one formulation do not necessarily supply relevant data on the absorption for all formulations. Inclusion of topical excipients not previously studied for adverse photoeffects in a new formulation may also warrant testing of the new formulation.
Testing should be conducted under conditions of simulated sunlight to be clinically relevant. Even though a particular substance has ground state absorption in UVA or UVB after it absorbs radiation, a transient or stable photoproduct may be produced that absorbs in a different absorption range (Becker et al., 1996; Navaratnam and Claridge 2000). A number of methods and approaches are used that test for photoirritation. Appropriate animal models (generally mice or guinea pigs, but also rabbits or swine) have been discussed by Marzulli and Maibach (1996, 1998) and Lambert et al. (1996). Several in vitro screens for photoirritation, such as the 3T3 neutral red uptake photocytotoxicity test, are available (Spielmann et al., 1998). The 3T3 assay may be useful for products that absorb UVA, UVB, or visible radiation. This assay may not be appropriate for the evaluation of some water-insoluble substances or complete drug formulations. Data from in vitro studies may provide sufficient information when conditions of the study are appropriate for the evaluation of the drug product of interest and may be important in planning more efficient comprehensive in vivo assessments.
For in vivo nonclinical studies, acute drug exposure followed by simulated sunlight exposure is generally considered adequate to identify potential risks. Assessments of photoirritation may be incorporated into ongoing general toxicity studies in some circumstances. Human studies are also often conducted to follow up on potential risks identified based on animal or in vitro evaluations.
Long-term photosafety testing is generally conducted only when it can provide useful information. Long-term photosafety studies should be avoided when sufficient information has already been collected for a drug or a class of drugs to appropriately inform potential users regarding photoreactivity.
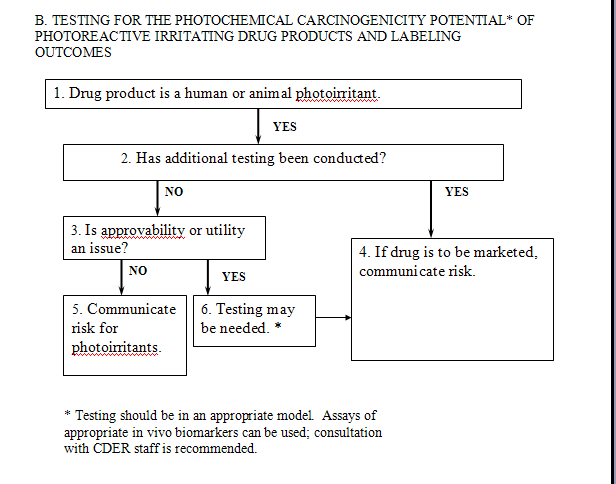
Once a systemically or dermally administered drug has been identified as a photoirritant in animal or human testing (see Flowchart A1), one should consider the drug’s potential to increase UV-associated skin cancer risk (Flowchart B). Because patients are already cautioned against excessive sunlight exposure during use of photoirritating drugs, sponsors could choose to strengthen these warnings with regard to photocarcinogenic potential, rather than conduct testing to determine the photochemical carcinogenicity potential for photoirritating drugs. The option to strengthen the warning statements without conducting additional testing would be appropriate primarily in those circumstances where photochemical carcinogenic activity would not affect approvability or significantly reduce the utility of a drug product. The warning statement should convey the basis of the warning and the conditions under which the potential carcinogenic effect is likely to be realized (see Box 5, Flowchart B).
Warnings alone may be sufficient because drug products that are photoirritants can cause rapid erythema (sunburn) reactions in patients who expose themselves to sun without adequate protection. Unlike many drug side effects, sunburn is immediately apparent to affected patients, who become quickly aware of the reactions during use. However, not all patients receiving a photoirritating drug may experience overt photoirrritation effects. Some drugs can cause subthreshold photoeffects (e.g., DNA damage) that are not apparent to patients. Thus, these drugs can also pose a long-term risk for adverse skin effects. It is important for product warnings to address this situation. Other circumstances for which product warning statements, rather than long-term testing, may be appropriate include the following:
· Drugs having structures significantly similar to known photochemical carcinogens
· Drugs that are in a known pharmacologic class of photochemical carcinogens where the pharmacology of the product is believed to be directly related to the carcinogenic potential
· Drugs for which several other tests for photoreactivity, such as in vitro photogenotoxicity, adduct formation, human photoirritation, or short-term in vivo nonclinical tests are positive
· Drugs that have been identified as carcinogens with potential human relevance in other assays that do not include UV sunlight, such as traditional two-year bioassays or transgenic assays
· Drugs for indications intended for populations in which the life expectancy is short (i.e., less than 5 years)
The warning should be informative, advising patients to avoid sun exposure, or if sunlight exposure cannot be avoided, to use protective clothing and broad-spectrum (UVA/UVB) sunscreens (when the wavelengths eliciting photoirritation are in the range covered by the sunscreen). However, it is important to recognize that subclinical photoirritation responses with prolonged use could also result in increased skin cancer risk. In general, for the above cases, warning statements are considered an adequate option, and phototesting, although potentially scientifically informative, may not be warranted. In those cases where additional testing may be of value, it can often be conducted during phase 4 of the drug development process (i.e., postapproval).
For drugs where the approvability or utility would be an issue (e.g., sunscreens), testing beyond that noted above may be appropriate. Testing should be conducted using a model for which there is evidence that relevant end points are assessed and considered scientifically valid (see Flowchart B, Box 6). In some circumstances, a drug sponsor may want to demonstrate that, despite initial results suggesting a potential for photocarcinogenicity, the drug does not pose a risk for UV-associated skin cancer. The results of appropriately conducted assays would be included in any communication of the overall risk (Boxes 4 and 5, Flowchart B).
Short-term assays that measure photoreactivity (such as photogenotoxicity) have been developed in the hope that they would provide information about the potential to enhance UV-induced skin carcinogenesis. However, the interpretation of such assays is not always straightforward, and their role in the evaluation of human risk should be carefully assessed. Although the most widely performed test for the potential to enhance UV-induced skin cancer is the hairless albino mouse model with solar simulation, other scientifically valid assays for evaluating the photochemical carcinogenicity potential can also be considered for regulatory purposes. When considering testing strategy, we encourage sponsors to discuss issues with the appropriate CDER review staff. One potential strategy is the use of biomarkers in human skin to evaluate the consequences of combined drug and UV exposure. Use of biomarkers should be considered and supported, based on a thorough evaluation of the scientific data (see section IV.C., Mechanistically Based and Other Assays).
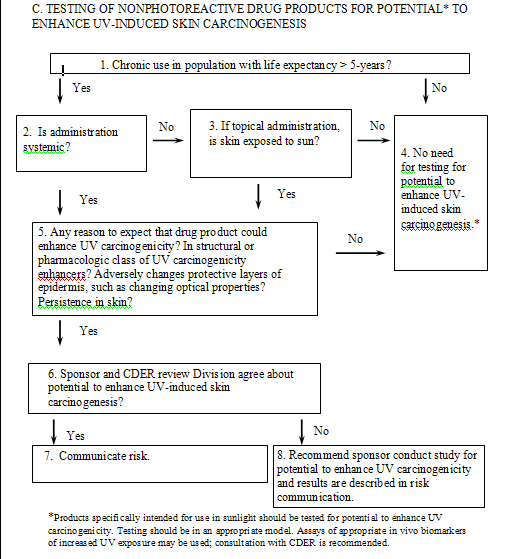
The decision-tree approach would apply to products used chronically or for chronic conditions as defined in the International Conference on Harmonization (ICH) guidance for industry S1B Testing for Carcinogenicity of Pharmaceuticals. As noted earlier, drug products that do not cause photoirritation reactions can enhance UV carcinogenicity. The decision tree used for nonphotoreactive products attempts to balance the risks associated with these potentially silent enhancers of UV-induced skin carcinogenesis, while attempting to identify areas where testing is unnecessary. Pharmacologic activity (see subsection IV.B.3. below) could provide information on such risks. It is anticipated that, even in the absence of information about such risks, most nonphotoreactive drugs would not be tested for potential to enhance UV-induced skin carcinogenesis, even if they were administered chronically. This assumes that when administered chronically, drugs usually would be tested for carcinogenicity in traditional bioassays. Some secondary mechanisms of enhancement of UV carcinogenicity, such as immunosuppression or inhibition of DNA repair, would be detected by use of traditional carcinogenicity studies.
The approach for nonphotoreactive drugs is described as follows (see also Flowchart C):
Nonphotoreacting drugs that are not used long term or for chronic conditions do not appear to present a significant risk of enhancing UV-induced skin carcinogenesis. Thus, it is unlikely that these drugs would be tested in any assay for potential to enhance UV-induced skin cancer. In addition, drug products intended solely for use in populations with a short life expectancy (less than 5 years) need not be tested. Chronic use may be continuous or substantial, repeated use and may justify such testing.
In general, topically applied drugs for which the intended effect is localized only to the area of application to non-sun-exposed skin and that do not reach pharmacologically measurable systemic levels will not need to be tested for potential to enhance UV-induced skin cancer. This principle also applies to other drugs that do not reach measurable systemic levels (e.g., drugs with mainly local effects on the respiratory tract).
The majority of drug products that are investigated and marketed are not photoreactive and are unlikely to be photococarcinogens. However, a major class of potent, known human photococarcinogens (e.g., immunosuppressants such as cyclosporin (Abel, 1989; Penn, 1988)) that cause skin neoplasms are nonphotoreactive. There are other examples of drug vehicles or nonphotoreactive drugs that enhance UV-induced skin carcinogenesis in mice (Jacobs et al., 1999; Learn et al., 2000). The mechanisms of enhancement by these nonphotoreactive drugs or vehicles have not been studied and can only be surmised. Some of the mechanisms by which nonphotoreactive vehicles or drugs can enhance UV-induced skin carcinogenesis include, but are not limited to, immunosuppression, neoplastic promotion, inhibition of apoptosis or DNA repair, and irritation, altering the protective layers of the epidermis and/or changing the optical properties of the skin. Such mechanisms are applicable to both rodent and human skin and are biologically plausible mechanisms of enhancement. Products, such as some emollients, which change the optical properties of the skin or alter the protective layers of the epidermis, can greatly change UV penetration of the skin or the effective UV dose that the skin receives. The open literature contains ample references to the effects of vehicles on skin and on the overall performance of a drug product. These and other indirect effects (discussed in section IV.C., Mechanistically Based and Other Assays) can also occur in human skin and may be as important as direct photoreactive effects. For example, studies sponsored by the cosmetics industry suggested increased sensitivity to UVB by persons using alpha-hydroxy acid preparations. As a consequence, the Cosmetic Ingredient Review Expert Panel (CIR 1998) recommended that persons using these products avoid unprotected exposure to the sun. The alpha-hydroxy acids used in these studies do not absorb UV between 280 and 400 nm. Thus, a thoughtful approach is called for when deciding if additional testing for potential to enhance UV-induced skin carcinogenesis is justified.
4. Warning or test (Boxes 6, 7, 8)
If preliminary evaluations suggest that a drug or drug product may have the potential to enhance UV-induced skin carcinogenesis, the sponsor should warn of this potential effect or conduct studies to evaluate this potential. Such studies could be a panel of appropriately selected and scientifically valid biomarkers in human skin, referred to in section IV.C., Mechanistically Based and Other Assays. Although some drug products that do not absorb light could lower the MED by changing the optical properties of the skin, resulting in increased UV effects, drugs that do not absorb light are not tested for photoirritation according to the current testing paradigm. If it were demonstrated that a nonphotoreactive drug product increased transmission of UV radiation through the skin, resulting in measurable increases in UV susceptibility, such as lowering the MED in animals or humans, further photosafety studies in animals, such as a photococarcinogenesis study, may not be appropriate. A product that increases the dose of UV penetrating the skin would likely shorten the time to skin neoplasms and could be labeled appropriately.
Mouse and human skin share many of the same responses to sunlight and drugs. Exposure to sunlight clearly modifies DNA and causes nonmelanoma skin cancer in both animals and humans (IARC, 1992). Although there are a number of differences, many of the proposed mechanisms by which drug substances or drug products can enhance UV-associated skin carcinogenesis are shared by mice and humans. Pyrimidine dimer formation and P53 protein induction have been demonstrated in human skin in situ after suberythemal doses of solar-simulated light (Burren et al., 1998). Evaluation of the potential to indirectly enhance UV carcinogenicity using biomarkers in skin may be appropriate, provided that the biomarkers are scientifically supported. A testing strategy can be discussed with the appropriate CDER review division. To improve testing procedures, it would be helpful to identify appropriate surrogate markers in human skin for increased UV exposure or UV damage.
Useful tests would be those that provide information on the relevance of, or sensitivity to, adverse photoeffects in vitro or in animals relative to humans. Tests could include, but would not be limited to, in vitro measures of photocytotoxicity, in vitro measures of photogenotoxicity (e.g., in Salmonella, yeast, or V79 cells), transgenic models, and biomarkers (molecular, biochemical, cellular, or structural) for enhancement of UV‑induced skin carcinogenesis in human skin. Changes in the MED, sunburn cell number (Lavker and Kaidbey, 1997), p53 alterations, dimer formation in DNA (Katiyar et al., 2000), and other end points have been proposed as markers of increased UVB exposure or skin damage. Markers for increased UVA exposure, as well as for UVB exposure, would be desirable. Although the preferred radiation exposure in these assays would be sunlight simulation, at a minimum, the appropriate absorption spectrum for a photoreactive drug product should be covered. Assays assessing immunosuppression or inhibition of DNA repair, particularly in human skin, may be useful in testing some products. It is important to define the strengths and limitations of the assays. Correlation of the in vitro results for photoirritation with data from controlled clinical studies would add to the potential utility of such tests. Correlation of the biomarker response in animal skin with the biomarker response in human skin for the same UV dose could provide a basis for evaluation of the size of a response in a clinical surrogate that would translate into a clinically meaningful increase in skin cancer risk. Submission of a test or rationale including relevant data should accompany any proposal to use novel methods.
The recommendations of this guidance recognize both the importance of adverse photoeffects and the difficulty in appropriately assessing human risks. This guidance allows a flexible approach to be used to address adverse photoeffects and does not require that a specific assay be used. Most important, it encourages the development of methods that can efficiently be used to evaluate human safety.
REFERENCES
Abel, E. A., 1989, “Cutaneous Manifestations of Immunosuppression in Organ Transplant Recipients,” J. Am. Acad. Dermatol. 21 (2 part 1): 167-179.
Anderson, R. R., and J. A. Parrish, 1981, “The Optics of Human Skin,” J. Invest. Dermatol. 77: 13-19.
Asker, A. F., and C. W. Harris, 1988, “Influence of Certain Additives on the Photostability of Physostigmine Sulfate Solutions,” Drug Development and Industrial Pharmacy 14 (5): 733-746.
Baynes, R. E., C. Browne, H. Freeman, and J. E. Riviere, 1996, “In Vitro Percutaneous Absorption of Benzidine in Complex Mechanistically Defined Chemical Mixtures,” Toxicol. Appl. Pharmacol. 141: 497-506.
Becker L., B. Eberlein-Konig, and B. Przybilla, 1996, “Phototoxicity of Non-steroidal Anti-inflammatory Drugs: In Vitro Studies with Visible Light,” Acta Derm. Venereol. 76 (5): 337-340.
Burren, R., C. Scaletta, E. Frenk, R. G. Panizzon, and L. A. Applegate, 1998, “Sunlight and Carcinogenesis: Expression of p53 and Pyrimidine Dimers in Human Skin Following UVA I, UVA I +II and Solar Simulating Radiations,” Int. J. Cancer 76: 201-206.
Chaquor, B., G. Bellon, S. Seite, J. P. Borel, and A. Fourtanier, 1997, “All Trans-Retinoic Acid Enhances Collagen Gene Expression in Irradiated and Non-Irradiated Hairless Mouse Skin,” J. Photochem. Photobiol. B: Biology 37: 52-59.
Chellquist, E. M., and W. G. Gorman, 1992, “Benzoyl Peroxide Solubility and Stability in Hydric Solvents,” Pharm. Res. 9 (10): 1341-1346.
CIR (Cosmetic Ingredient Review), 1998, “Final Report on the Safety Assessment of Glycolic Acid, Ammonium, Calcium, Potassium, and Sodium Glycolate, Methyl, Ethyl, Propyl, and Butyl Glycolate, and Lactic Acid, Ammonium, Calcium, Potassium, Sodium, and TEA‑ Lactate, Methyl, Ethyl, Isopropyl, and Butyl Lactate, and Lauryl, Myristyl, and Cetyl Lactate,” Int. J. Toxicol. 17(Suppl. 1): 1-241.
Dearman, R. J., M. Cumberbatch, J. Hilton, H. M. Clowes, I. Fielding, J. R. Heylings, and I. Kimber, 1996, “Influence of Dibutyl Phthalate on Dermal Sensitization to Fluorescein Isothiocyanate,” Fundam. Appl. Pharmacol. 33: 24-30.
Frezza, E. E., J. Fung, and D. H. van Thiel, 1997, “Non-lymphoid Cancer After Liver Transplantation,” Hepatogastroenterology 44 (16): 1172-1181.
Gibbs, N. K., A. R. Young, and I. A. Magnus, 1985, “Failure of UVR Dose Reciprocity for Skin Tumorigenesis in Hairless Mice Treated With 8-Methoxypsoralen,” Photochem. Photobiol. 42 (1): 39-42.
Hessel, A., R. J. Siegle, D.L. Mitchell, and J. E. Cleaver, 1992, “Xeroderma Pigmentosum Variant With Multisystem Involvement,” Arch. Dermatol. 128 (9): 1233-1237.
Holzle, E., N. Neumann, B. Hausen, B. Przybilla, S. Schauder, H. Honigsmann, A. Bircher, and G. Plewig, 1991, “Photopatch Testing: The 5 Year Experience of the German, Austrian, and Swiss Photopatch Test Group,” J. Am. Acad. Dermatol. 25: 59-68.
IARC, 1992, “Solar and Ultraviolet Radiation,” IARC Monographs on the Evaluation of Carcinogenic Risk to Humans, Vol. 55, IARC, WHO.
ICH guidance for Industry S1B Testing for Carcinogenicity of Pharmaceuticals, available on the Internet at http://www.fda.gov/cder/guidance/index.htm.
Islam, M. S., and A. F. Asker, 1995, “Photoprotection of Daunorubicin Hydrochloride With Sodium Sulfite,” PDA J. Pharmaceut. Sci. Technol. 49 (3): 122-126.
Jacobs, A., J. Avalos, P. Brown, and J. Wilkin, 1999, “Does Photosensitivity Predict Photococarcinogenicity?” Internat. J. Toxicol. 187 (4): 191-198.
Johnson, B. E., N. K. Gibbs, and J. Ferguson, 1997, “Quinolone Antibiotic With Potential to Photosensitize Skin Tumorigenesis,” J. Photochem. Photobiol. B: Biology 37: 171‑173.
Johnson, B. E., 1984, “Light Sensitivity Associated With Drugs and Chemicals,” Physiol. Pathophysiol. Skin 8: 2542-2606.
Kaidbey, K., and A. Kligman, 1974, “Topical Photosensitizers: Influence of Vehicles on Penetration,” Arch. Dermatol. 110: 868-870.
Katiyar, S. K., M. S. Matsui, and H. Mukhtar, 2000, “Kinetics of UV Light-induced Cyclobutane Pyrimidine Dimers in Human Skin in Vivo: An Immunohistochemical Analysis of Both Epidermis and Dermis,” Photochem. Photobiol. 72(6): 788-793.
Kochevar, I. E., M. A. Pathak, and J. A. Parrish, 1993, “Photophysics, Photochemistry, and Photobiology,” Dermatology in General Medicine, Fitzpatrick, T. B., A. Z. Eisen, K. Wolff, I. M. Freedburg, K.F. Austen, Eds,. Fourth ed., McGraw-Hill, New York, pp. 1627-1638.
Kornhauser, A., W. G. Wamer, and L. A. Lambert, 1996, “Cellular and Molecular Events Following Ultraviolet Irradiation of Skin,” in Dermatotoxicology, Fifth ed., Taylor and Francis, Washington, DC, pp. 189-230.
Kraemer, K. H., M. M. Lee, A. D. Andrews, and W.C. Lambert, 1994, “The Role of Sunlight and DNA Repair in Melanoma and Nonmelanoma Skin Cancer - The Xeroderma Pigmentosum Paradigm,” Arch. Dermatol. 130 (8): 1018-1021.
Lambert, L. A., W. G. Wamer, and A. Kornhauser, 1996, “Animal Models for Phototoxicity Testing,” in Dermatotoxicology, Fifth ed., Marzulli, F. N., and H. I. Maibach, eds., Taylor and Francis, New York, pp. 515-529.
Lavker, R., and K. Kaidbey, 1997, “The Spectral Dependence for UVA-induced Cumulative Damage in Human Skin, ” J. Invest. Dermatol. 108(1): 17-21.
Learn, D. B., C. P. Sambuco, P. D. Forbes, and A. M. Hoberman, 2000, “Phototoxicology: Photocarcinogenesis Historical Control Data as a Key Interpretative Element,” The Toxicologist 54 (No. 1, pt. 2): 145.
Lindahl, T., P. Karran, and R. D. Wood, 1997, “DNA Excision Repair Pathways,” Curr. Opin. Genet. Dev. 7(2): 158-169.
Marti-Mestres, G., G. Fernandez, N. Parsotam, F. Nielloud, J. P. Mestres, and H. Maillols, 1997, “Stability of UV Filters in Different Vehicles: Solvents and Emulsions, Drug Development Industry,” Pharmacy 23 (7): 647-655.
Marzulli, F. N., and H. I. Maibach, 1991, Dermatotoxicology,Fourth ed., Marzulli, F. N., and H. I. Maibach, eds., Taylor and Francis, New York, p. 585.
Marzulli, F. N., and H. I. Maibach, 1996, “Photoirritation (Phototoxicity, Phototoxic Dermatitis),” Dermatotoxicology, Fifth ed., Marzulli, F. N., and H. I. Maibach, eds., Taylor and Francis, New York, p. 231-237.
Marzulli, F. N., and H. I. Maibach, eds., 1998, Dermatotoxicology Methods, Taylor and Francis, New York.
Megaw, J. M., and L. A. Drake, 1986, Photobiology of the Skin and Eye, Marcel Dekker: New York.
Moan, J., A. Dahlback, and R. B. Setlow, 1999, “Epidemiological Support for an Hypothesis for Melanoma Induction Indicating a Role for UVA Radiation,” Photochem. Photobiol. 70 (2): 243-247
Navaratnam S., and J. Claridge, 2000, “Primary Photophysical Properties of Ofloxacin,” Photochem. Photobiol. 72(3): 283-290.
Penn, I., 1988, “Tumors of the Immune Compromised Patient,” Ann. Rev. Med. 39: 63-73.
Physicians’ Desk Reference, 2000, 54th ed., Medical Economics Co., Montvale, NJ.
Serup, J., A. Winther, and C. Blichmann, 1989, “A Simple Method for the Study of Scale Pattern and Effects of a Moisturizer--Qualitative and Quantitative Evaluation by D-Squame Tape Compared With Parameters of Epidermal Hydration,” Clin. Experiment. Dermatol. 14: 277-282.
Smith, K. C., Ed., 1989, The Science of Photobiology, 2nd ed., Plenum Press: New York.
Spielmann, H., M. Balls, J. Dupuis, W. J. Pape, G. Pechovitch, et al., 1998, “The International EU/COLIPA in Vitro Phototoxicity Validation Study: Results of Phase II (Blind Trial): Part 1: The 3T3 NRU Phototoxicity Test,” Toxicology in Vitro 12 (3): 305-327.
Stern, R. S. , and E. J. Lunder, 1998, “Risk of Squamous Cell Carcinoma and Methoxsalen (Psoralen) and UV-A Radiation (PUVA). A Meta-Analysis, ” Arch. Dermatol. 134 (12): 1582-1585.
Wrench, R., 1980, “Epidermal Thinning: Evaluation of Commercial Corticosteroids,” Arch. Dermatol. Res. 267: 7-24.
GLOSSARY
ADR: Adverse drug reaction
IR: Infrared radiation 0.76 mm- 1000 mm
MED: Minimal erythema dose
8-MOP: 8-Methoxypsoralen
NSAID: Nonsteroidal anti-inflammatory drug
Excipients: Ingredients that are intentionally added to therapeutic products but that do not directly exert pharmacologic effects at the intended dosage
Indirect photoeffects: Effects of an agent, vehicle, or product on the optical, structural, molecular, or physiologic properties of the skin, such that the interaction of light and skin or effects of drug in skin are altered.
Nonphotoreactive: Drugs or chemicals that do not react with another molecule in the formulation or skin after exposure to UVA, UVB, or visible radiation
Photoallergy: An acquired, immunologically mediated reaction to a drug or chemical initiated by the formation of photoproducts when that drug or chemical is exposed to light
Photochemical carcinogenesis: Carcinogenesis resulting from a reaction with a photoactivated drug or chemical
Photococarcinogenicity: The direct (photochemical carcinogenesis) or indirect enhancement of UV-associated skin carcinogenesis (e.g., sunlight-associated carcinogenesis) by a drug or chemical
Photoirritation or Photochemical irritation: A light‑induced, nonimmunologic, skin response to a photoreactive drug or chemical
Photoproducts: Compounds resulting from absorption of radiation by a drug or chemical
Photoreactive: Drugs or chemicals that react with another molecule in the formulation or skin after exposure to UVA, UVB, or visible radiation
Photosafety testing: Testing for the potential of a drug product to cause photoirritation or photoallergy or to enhance UV-induced skin carcinogenesis
Photosensitivity: A photoirritation- or photoallergy-induced reaction
Photosensitizer: A drug or chemical that causes an adverse effect in the presence of UVA/UVB or visible light
Phototoxicity: A light‑induced, nonimmunologic response to a photoreactive drug or chemical
PUVA: Psoralen plus UVA treatment
UV: Ultraviolet radiation (wavelengths between 10 and 400 nm)
UVA: Ultraviolet radiation A (wavelengths between 320 and 400 nm)
UVB: Ultraviolet radiation B (wavelengths between 290 and 320 nm)
UVC: Ultraviolet radiation C (wavelengths between 200 and 290 nm)
This guidance has been prepared by the Pharmacology Toxicology Coordinating Committee in the Center for Drug Evaluation and Research (CDER) at the FDA.








