Clinical Research in Chronic Obstructive
Pulmonary Disease
Needs and Opportunities
NHLBI Workshop Summary
Published in Am J Respir Crit Care Med Vol 167. pp
1142-1149, 2003 Internet address: www.atsjournal.org
Thomas L. Croxton, Gail G. Weinmann, Robert M.
Senior, Robert A. Wise, James D. Crapo, and A. Sonia Buist
Division of Lung Diseases, National Heart, Lung, and
Blood Institute, Bethesda, Maryland; Departments of Medicine and of Cell
Biology and Physiology, Washington University School of Medicine and
Barnes-Jewish Hospital, St. Louis, Missouri; Department of Medicine, The Johns
Hopkins University, Baltimore, Maryland; Department of Medicine, National
Jewish Medical and Research Center, Denver, Colorado; and Department of
Medicine, Oregon Health and Science University, Portland, Oregon
Chronic obstructive pulmonary disease (COPD) is a
common condition, and one difficult to manage. Available treatments, other than
smoking cessation, are only minimally effective, and the knowledge basis for
clinical decision making is limited. To identify areas in which further
clinical research may lead to significant improvements in the care of patients
with COPD, the National Heart, Lung, and Blood Institute convened a Working
Group, entitled "Clinical Research in COPD: Needs and Opportunities," on March
21-22, 2002. This group of experts identified important questions in the field
and made the following recommendations: (1 ) establish a multicenter Clinical
Research Network to perform multiple, short-term clinical trials of treatments
in patients with moderate-to-severe COPD; (2 ) create a system for the
standardized collection, processing, and distribution of lung tissue specimens
and associated clinical and laboratory data; (3 ) develop standards for the
classification and staging of COPD; (4 ) characterize the development and
progression of COPD using measures and biomarkers that relate to current
concepts of pathogenesis; and (5 ) evaluate indications for long-term oxygen
therapy for patients with COPD.
Keywords: chronic obstructive pulmonary
disease; lung diseases, obstructive; National Institutes of Health
Chronic obstructive pulmonary disease (COPD) causes
more than 500,000 hospitalizations and more than 100,000 deaths in the United
States each year (1, 2). In addition, millions of Americans are disabled as a
result of this disease. Unfortunately, the treatment options available to
patients with COPD and their physicians are limited, and no pharmacologic
therapy slows the progressive loss of lung function that occurs. Smoking
cessation slows the decline in FEV1, but the sustained quit rates attained by
intensive smoking cessation interventions are low. Long-term oxygen
therapy is the only other treatment that has been shown to improve survival,
but oxygen appears to extend life by less than 2 years in patients with
advanced disease (3).
Because of the health burden imposed by COPD and the
urgent need for better management of this disease, the National Heart, Lung,
and Blood Institute (NHLBI) convened a Working Group on March 21-22, 2002 to
examine needs and opportunities for clinical research in COPD. This article
summarizes background information considered by the Working Group, lists
important questions that were raised, and reports specifc recommendations.
Recommendations from a complementary Working Group, focused primarily on basic
science issues related to COPD, were reported previously (4).
STATUS OF COPD IN THE UNITED STATES
What is COPD? Issues of Diagnosis and Awareness
COPD is usually defined in terms of physiology--a condition of airflow
limitation that is due to both airway and airspace disease, is relatively
stable , and is only partially alleviated by bronchodilator drugs (5). The
airflow limitation typically progresses slowly over time. Other descriptions
emphasize specific aspects of the COPD spectrum (e.g., chronic bronchitis or
emphysema) or clinical presentation (e.g., chronic dyspnea, usually in a
long-term smoker with productive cough). The phrase "abnormal inflammatory
response of the lung to noxious particles or gases" found in the NHLBI/WHO
Global Initiative for Chronic Obstructive Lung Disease definition of COPD may
portend future emphasis on pathogenetic mechanisms in descriptions of COPD (6).
A profusion of terminology used with COPD causes
confusion among patients, if not among physicians. Many patients diagnosed with
smokers' lung, emphysema, bronchitis, chronic bronchitis, chronic obstructive
bronchitis, or obstructive lung disease do not even realize that they have
COPD. This multiplicity of names complicates epidemiologic studies of COPD:
self report of physician diagnosis is a poor measure of COPD prevalence, and
reports of impaired lung function from the National Health and Nutrition
Examination Survey (NHANES) III carefully defined "obstructive" but included in
the analysis those in whom the impairments may not have been "chronic" (e.g.,
viral infection), "pulmonary" (e.g., congestive heart failure), or "disease"
(e.g., normal subjects with outlier laboratory values) (7). Although the risk
of lung cancer is common knowledge, few smokers recognize "COPD" as a threat
that is nearly as likely to kill them and far more likely to severely disable
them.
Burden of Disease in the United States
Despite limitations of the epidemiologic data, it is
abundantly clear that COPD is a tremendous public health problem whose risk
factors, in particular genetic risk factors, are poorly understood. COPD
typically occurs insidiously in individuals with a long history of cigarette
smoking, which usually begins at about age 15. Who will and who will not
develop COPD cannot usually be ascertained until middle age, and an additional
15 years may pass between the onset of detectable disease and physician
diagnosis of COPD. Only about 15% of chronic smokers develop clinically
significant COPD, and fewer than 15% of these are diagnosed with emphysema (5).
Significant factors for disease risk within the population of smokers have not
been identified. On the other hand, about 15% of cases are not attributable to
cigarettes, and the causes of disease in this minority are poorly characterized
(5). COPD afflicts more than 15 million Americans, results in more than 15
million physician office visits each year, and causes approximately 150 million
days of disability per year (8-10). The total direct cost of medical care
related to COPD is approximately $15 billion per year (11).
This dire situation is not expected to improve for
years. COPD has risen to become the fourth leading cause of death in the United
States (2). Fewer than half of those in this country with airflow limitation
that can be detected by spirometry have been diagnosed with an obstructive lung
disease, and the remainder are presumably unaware of their high risk for
progressive loss of lung function (7). Only a fourth of willing participants in
smoking cessation programs become sustained quitters, and the prevalence of
current smoking is fairly constant at one fourth of American adults (12).
Especially discouraging is the fact that a fourth of high school students smoke
(13). Each day, as nearly 300 of their forebears die, 6,000 children try their
first cigarette and 3,000 advance to daily smoking (14).
Medical and Surgical Management of COPD
The only intervention of proven value in early-stage
COPD is smoking cessation. The Lung Health Study demonstrated that those
randomized to a smoking cessation program had better pulmonary function 11
years later than those who received usual care, and the rate of decline in FEV1
in sustained quitters was similar to that seen in nonsmokers (15).
Unfortunately, smoking interventions achieve long-term cessation in only a
minority of participants. It is not yet known if the original intensive smoking
cessation intervention will alter mortality in the Lung Health Study cohort
(intent to treat analysis).
The only medical therapy shown to improve survival in
COPD is long-term oxygen administration to individuals with advanced disease
and low arterial oxygen levels. Controlled clinical trials performed more than
20 years ago demonstrated enhanced survival with oxygen supplementation in
those with severe arterial hypoxemia (3, 16). This effect was greatest in those
for whom oxygen was prescribed 24 hours per day. No comparable studies have
satisfactorily assessed the value of oxygen therapy in those with less severe
hypoxemia or with isolated nocturnal or exercise-related oxyhemoglobin
desaturation, although supplemental oxygen is often used in these
situations.Furthermore, possible effects of oxygen supplementation on quality
of life, depression, and cognitive function have not been well studied.
Bronchodilators are commonly used in COPD to provide
symptomatic relief, but they do not retard the progression of the disease
as measured by decline in FEV1 (5). Anticholinergics, long-and short-acting
beta-adrenergic agonists, or formulations combining these classes of drugs,
appear to decrease dyspnea, increase FEV1, decrease the frequency of reported
exacerbations, and improve quality of life (17, 18). Theophylline may be of
benefit in some patients, even in the absence of measurable bronchodilation
(19).
Regular use of inhaled corticosteroids may reduce
symptoms, frequency of exacerbations, and numbers of outpatient physician
visits in patients with moderate or severe COPD, but does not affect the rate
of decline in postbronchodilator FEV1 (20, 21). Courses of systemic
corticosteroids begun during an acute exacerbation appear to speed the recovery
of lung function, reduce the length of hospitalization, and decrease the
frequency of treatment failure (22, 23). However, chronic use of systemic
corticosteroids does not improve the course of COPD, and may increase mortality
(6, 24).
Antibiotics are often given for exacerbations of COPD,
in part because of an association of bacterial infection with
exacerbations in some cases (25). Clinical trials examining the effects
of antibiotics have shown benefits, albeit inconsistently, with respect to peak
expiratory flow rate and duration of exacerbation (26, 27). The benefit of
antibiotics appears to be greatest in those with more severe exacerbations or
more severe disease. It is noteworthy that most studies of antibiotics were
performed 30-45 years ago and that the antibiotics most commonly used today for
exacerbations have not been tested in clinical trials for COPD.
Because excess production of mucus is a prominent
feature in many patients with COPD, there is longstanding interest in drugs
that regulate mucous secretions. Experts disagree with regard to the clinical
value of mucolytic agents in COPD. The ATS Statement of 1995 allowed, but did
not encourage, the use of mucokinetic agents, and the recent Global Initiative
for Chronic Obstructive Lung Disease guidelines judged the overall effects of
these drugs to be small, and did not recommend their widespread use (5, 6).
However, a recent Cochrane Review meta-analysis found significant effects of
mucolytics in reducing exacerbations in stable chronic bronchitis and COPD
(28). Because none of the available drugs is a particularly effective
mucoregulatory agent, the real potential of this general therapeutic approach
has yet to be tested.
Pulmonary rehabilitation is a multidisciplinary
intervention that combines an exercise program with behavioral, psychosocial,
and educational support. There is strong evidence that such programs increase
exercise tolerance and decrease dyspnea, and there is weaker evidence for
improvements in quality of life and use of health care resources (29, 30). No
significant increase in survival has yet been demonstrated; longer-term results
from short-term programs have shown inconsistent results.
Lung transplantation is a conceptually attractive
treatment for COPD, but is accessible to few patients because of the small
number of available donor organs and limited resources. Limited data indicate
that transplantation improves pulmonary function, exercise capacity, and
quality of life, but studies are inconsistent with regard to effects on
survival (31-35). Lung volume reduction surgery for emphysema is reported to be
beneficial in some patients (36). The ongoing National Emphysema Treatment
Trial is designed to measure the efficacy of this procedure in individuals with
severe emphysema and to identify characteristics of those patients most likely
to benefit from it (37). Results are expected in 2003. New surgical approaches
to treatment are being investigated in animal models of emphysema.
Potential for Novel Therapies
Given the modest benefits from current treatment
modalities, substantial progress in COPD treatment may require the development
of entirely new therapeutic approaches. Recent advances in understanding of
pathophysiologic processes that may underlie this disease suggest six possible
approaches. The first derives from the early discovery that deficiency of the
protease inhibitor alpha1-antitrypsin is one cause of emphysema (38). Recent
attention has focused on matrix metalloproteinases, which are released from a
variety of lung and inflammatory cells and may play a key role in the
pathogenesis of COPD (39). Notably, transgenic mice deficient in macrophage
elastase were resistant to cigarette smoke-induced airspace enlargement (40).
Hence, there is hope that inhibition of specific matrix metalloproteinases,
inhibition of serine proteases that inactivate inhibitors of the matrix
metalloproteinases, or reduction of matrix metalloproteinase expression
through transcriptional regulation might slow or prevent the development or
progression of COPD. A theoretical concern with this approach is that
interfering with some of these proteases may increase the risks of infection or
cancer.
A second approach would be to counter the effects of
elastic fiber degradation by enhancing the synthesis, rate of assembly, or
stability of elastic fibers in the lung. Elastic fiber synthesis is a complex
process, and little is known about it in the context of emphysema. The role(s)
of the microfibrillar components of the fibers has received little attention.
Moreover, the linkage of elastic fibers to cells via fibulin-5 appears to be
crucial to preservation of the fibers, because transgenic mice deficient in
fibulin-5 developed severely disorganized elastic fibers and emphysema (41,
42). The process of elastic fiber assembly may admit both vulnerabilities and
opportunities for therapeutic manipulation.
A third approach would be to prevent that portion of
matrix injury due to inflammatory cell products by inhibiting the recruitment
of inflammatory cells to the lung. Failure of corticosteroids to alter the
course of COPD does not disprove this concept, because corticosteroids are
relatively ineffective against neutrophilic inflammation and have other actions
that might override a beneficial antiinflammatory effect (43). Implementation
of this approach may first require detailed characterization of the
inflammatory process throughout the natural history of COPD, identification of
factors that exaggerate the inflammatory process (such as latent adenoviral
infection [44]), and development of selective drugs that target relevant
inflammatory pathways or cells.
A fourth approach is enhancement of the antioxidant
capabilities of the lung. Several observations indicate that oxidants may
function as mediators of COPD pathogenesis: (1 ) substantial oxidative stress
derives both from cigarette smoke and from neutrophils; (2 ) oxidants produce a
multitude of relevant biological effects, including enhancement of neutrophilic
inflammation, secretion of mucous, activation of matrix metalloproteinases,
inactivation of alpha1-antitrypsin, and inhibition of glucocorticoid responses
(45-47); (3 ) biomarkers of oxidative stress are increased in the breath of
subjects with stable COPD, including those who have never smoked (48); and (4 )
observational studies suggest a beneficial effect of dietary antioxidants on
pulmonary function (49). Prospective data are needed to determine if exogenous
antioxidants can prevent COPD or slow its progression.
A fifth possible approach is based on the observations
that (1 ) induction of apoptosis of pulmonary endothelial cells in experimental
animals caused emphysema, and (2) increased numbers of apoptotic cells were
observed in the alveolar septae of emphysematous human lungs (50, 51). If, as
these data suggest, apoptotic death of alveolar cells plays a role in the
pathogenesis of emphysema, pharmacologic inhibition of apoptosis might prevent
loss of alveoli. Related possibilities for therapy may exist in other pathways
involved in physiologic maintenance of lung structure or in lung growth during
development. An example of the latter is the possible use of retinoids to
stimulate alveolarization (52).
A sixth novel approach to COPD treatment would be to
decrease the production of mucus by regulation of goblet and glandular mucous
cells. Many possible targets for novel therapies exist, including chemical
mediators of mucous secretion or mucous metaplastic transformation, growth
factors for cellular hyperplasia, and signal transduction pathways involved in
mucin gene expression.
Much remains to be learned about the disease process
in COPD, and other novel approaches to COPD treatment may be forthcoming in the
next few years. Vigorous evaluation of all possibilities is important, because
experts in COPD do not expect any single agent or approach to be sufficient in
itself for the prevention or treatment of COPD. Rather, it is thought that a
combination of drugs will be required for adequate control of this complex
disease.
IMPORTANT CLINICAL QUESTIONS IN COPD
While considering what is currently known, the Working
Group identified a number of specific deficits in knowledge that limit
improvements in the clinical management of COPD. These deficits range from
basic understanding of the disease process to uncertainties in the use and
evaluation of existing treatments to questions of how to better develop and
test new therapies. This section describes 14 questions raised by the Working
Group, which convey these important deficits of knowledge and which underlie
the recommendations developed by the group.
1. What Changes Occur in the Lung Early in the
Development of COPD?
Little is known about early changes, before the onset
of significant airflow limitation, that occur in the lungs of smokers who will
develop COPD. Research is needed to identify molecular, cellular, structural,
and functional changes in the lungs of smokers, with and without COPD, across a
wide range of ages. A companion need is for the identification and validation
of biomarkers that correlate with disease activity and can be related to
specific biochemical pathways.
2. Is Early Diagnosis of Value?
Spirometry offers a sensitive and inexpensive means of
detecting COPD long before the stage at which most patients seek medical
attention. However, few primary care physicians perform routine spirometry,
even in those smokers over age 45 for whom it has been recommended (53). Hence,
COPD is greatly underdiagnosed. Because no therapy other than smoking cessation
is known to alter the course of mild or moderate COPD, the strongest rationale
for early detection is the possibility that a patient's knowledge of disease
(i.e., low FEV1) might enhance smoking cessation efforts. Existing studies of
the influence of spirometric testing on quit rate are inconsistent (54, 55).
Additional clinical trials, which take into account the covariance of FEV1 and
nicotine dependence (56), are needed to determine if spirometric testing can
augment smoking cessation interventions.
3. Can the Heterogeneous COPD Population Be Divided
into More Homogenous Subgroups on the Basis of Clinical Features and Laboratory
Measures?
COPD is a protean condition whose diverse
presentations include centriacinar emphysema, panacinar emphysema, and chronic
bronchitis without appreciable emphysema (5). Furthermore, a host of clinical
and laboratory measurements are often abnormal in COPD, and some of these show
both great variability among patients and weak correlation with other measures.
Various biomarkers have been identified, and certain genotypes have been
associated with the disease (43, 57). Although a rich array of parameters is
available for stratification of patients with COPD, few of these (e.g., FEV1,
methacholine responsiveness, radiographic indices of emphysema) are commonly
used to predict outcome. Guidelines for the selection of therapies are
generally based on unidimensional scales of severity (5, 6). An important
challenge in COPD is the development of more powerful, multivariate
methods for predicting individual outcome and individual responsiveness to
particular therapies on the basis of clinical and laboratory characteristics.
4. What Is the Natural History of COPD during its
Later Stages?
Much of our knowledge of the natural history of COPD
comes from cross-sectional studies of symptoms and spirometry in occupational
cohorts performed more than a quarter century ago. Those studies provided
little information about the late stages of COPD. Furthermore, they were
performed outside the context of current medical management (e.g., long-term
oxygen therapy), without modern measures of the disease (e.g., CT assessment of
emphysema), and with little emphasis on exercise limitation as an important,
quantifiable manifestation of severe disease. Improvements in clinical care for
those with severe COPD would be aided by longitudinal studies of late stage
disease that correlate advanced measures of the disease with outcome.
5. What Is the Pathogenetic Relationship between
COPD and Lung Cancer?
Epidemiologic studies have demonstrated comorbidity of
COPD and lung cancer in excess of that attributable to smoking, suggesting that
these conditions may share genetic risk factors or involve common pathogenetic
mechanisms (58). For example, inflammatory mediators, including oxidants and
NO, that may be important in COPD can also induce DNA damage, inhibit DNA
repair, and chemically activate carcinogens (59, 60). Identification of common
molecular processes in COPD and lung cancer could have important implications
for the prevention and management of both diseases. Human, animal, and in vitro
studies are needed to investigate cellular and molecular mechanisms common to
COPD and lung cancer. There is also rationale for unified clinical studies of
COPD and lung cancer in the areas of genetic susceptibility and
chemoprevention.
6. What Measures of Disease Status Are Useful
Indices of Therapeutic Benefit?
Demonstration of airflow limitation (e.g., decreased
FEV1) is essential for diagnosis and is the best known predictor of outcome in
COPD. Hence, decline in postbronchodilator FEV1 over time has been used as the
"gold standard" measure of disease progression in premorbid COPD. However,
emerging evidence indicates that alternative measures, such as inspiratory
capacity, may better reflect the ventilatory dysfunction in COPD (61, 62).
Furthermore, reliance on FEV1 may cause studies to miss beneficial effects of
therapies such as increased exercise capacity, quality of life, or cognitive
function, or lessened dyspnea, cough, sputum production, depression, or
frequency or severity of exacerbations (63). Alternative measures are needed
that better reflect the clinical status of patients with COPD and allow
detection of clinically important responses to therapies. It is noteworthy
that, in a trial of alpha1-antitrypsin augmentation in patients deficient in
this protein, progression of emphysema could apparently be detected in less
time by measurement of lung density using computed tomography than by pulmonary
function testing (64).
7. How Can Exacerbations Be Better Managed?
Acute exacerbations of COPD are the major battle front
of the physician's war on this disease, and the arsenal is ineffective (65).
Current treatment consists primarily of supportive measures in combination with
drugs appropriated from the pharmacopoeias of asthma and pneumonia, which have
limited effectiveness in COPD (65). Although such treatments are of some
benefit, nearly half of patients with COPD hospitalized for severe
exacerbations are dead within a year (66). Controlled studies are needed to
rigorously evaluate the efficacy of current management approaches and to refine
the indications for existing drugs. Methods of mechanical ventilation can
likely be improved with better understanding of how patients with severe
exacerbations of COPD respond physiologically to critical care interventions.
New pharmacologic agents are needed, especially drugs that are capable of
controlling the excess production and/or retention of mucus within the airways.
In addition, greater emphasis is needed on the prevention of exacerbations,
because this approach may do much to extend life and reduce the costs of care
for patients with COPD.
8. Who Should Get Long-Term Oxygen Therapy and
When?
Although oxygen supplementation relieves hypoxemia, it
may increase oxidative stress, an insult thought to be involved in the
pathogenesis of COPD (59). Hence, the value of oxygen treatment in COPD should
be determined by clinical trials of sufficient duration to detect effects on
mortality. Although there is clear value for long-term oxygen in those with
resting PaO2 <= 55 mm Hg (3), there have not been adequate trials to assess
the benefit of this treatment in other groups (67). Studies are needed in those
with moderate hypoxemia, those with nocturnal oxyhemoglobin desaturation, and
those who desaturate with ambulation. Possible effects of oxygen therapy on
cognitive function and quality of life need to be assessed.
9. How Can Exercise Capacity Be Increased?
Exercise limitation is prevalent in COPD and is
predictive of mortality among those with severe disease (68, 69). The
physiologic basis of this limitation is multifactorial (70). Ventilatory
impairment, sensation of dyspnea, cardiopulmonary interactions, skeletal and
respiratory muscle dysfunction, and general systemic illness may all contribute
in certain patients. A better understanding of the proximate causes of
diminished exercise capacity is important for improvements in pulmonary
rehabilitation programs and for the development of new therapeutic strategies
that may enhance physical performance in those with COPD. Of particular
interest are the origin and treatment of skeletal muscle dysfunction, a
well-documented systemic manifestation of COPD that may be amenable to
pharmacologic interventions (71).
10. How Can Nutritional Status Be Improved?
Weight loss is often observed in severe COPD, and low
body mass index is an independent predictor of respiratory mortality among
those with COPD (72). There is no satisfactory explanation for why some, but
not all, individuals with severe COPD lose weight, and there is no accurate
method for predicting who will or will not become cachectic. Understanding the
mechanisms of COPD-associated cachexia would be helpful for the design of a
rational therapy for this condition. Although it is thought that weight loss in
COPD is mainly due to diminished food intake rather than increased metabolism,
trials of caloric supplementation have generally been disappointing. A
meta-analysis of selected studies demonstrated increases in weight, but no
significant improvement in other measures of disease severity (73). Nutritional
supplementation in combination with exercise and/or anabolic drugs has not been
adequately tested.
11. Is Control of Sleep Disorders an Important
Aspect of COPD Management?
Sleep disturbance is common in COPD, and may
contribute to depression, cognitive dysfunction, and lessened quality of life
in this disease (74). Effective treatments of sleep disturbance in individuals
with COPD are needed that minimize the respiratory depressant effects of many
hypnotic drugs (75). In addition, COPD can coexist with obstructive sleep
apnea, compounding the ventilatory defect of each condition (76). Studies are
needed to evaluate the use of oxygen or noninvasive ventilatory support in
patients with this "overlap syndrome."
12. How Should Comorbid Conditions Be Managed?
Patients with COPD are at enhanced risk of associated
comorbidities such as cardiovascular disease, lung cancer, and sleep-disordered
breathing (58, 74). Despite this, little research has been done to determine
the optimal means of managing COPD in combination with other conditions. For
example, there is uncertainty as to whether COPD should be a relative
contraindication for the use of beta-adrenergic blockers in patients with heart
disease (77); and denials of lung cancer resection because of low FEV1 may
unnecessarily discriminate against those with COPD (78). Studies are needed to
improve management strategies for those with coexisting diseases.
13. What Can Be Done to Promote the Development and
Testing of Novel Agents for the Treatment of COPD?
Several factors impede the development and testing of
novel treatments for COPD. First, because the key pathogenetic pathways are not
established, financial incentives for pharmaceutical companies favor trials of
drugs already used for other diseases, rather than de novo development of
targeted agents for COPD. Second, the unavailability of validated surrogate
markers of COPD makes studies to establish proof of principle or appropriate
dosage both complex and expensive. Third, the slow progression of COPD requires
that efficacy trials be of long duration. Fourth, the heterogeneity of COPD
requires large numbers of subjects for clinical trials of therapeutics. Efforts
that may reduce these barriers to the development of novel agents include basic
research on COPD pathogenesis; investigations of surrogate endpoints and
indices for therapeutic stratification; exploration of alternative outcome
measures; and greater cooperation among research institutions, funding
agencies, health care providers, regulatory agencies, pharmaceutical companies,
and health care payors in the conduct of clinical studies. For some drugs,
there may be advantages to initial testing in patients with alpha1-antitrypsin
deficiency because of the more rapid decline of FEV1 in this group than in
usual COPD. The Alpha-1 Research Registry, now maintained by the Alpha One
Foundation and the Medical University of South Carolina (Charleston, SC),
contains demographic and clinical data on individuals with severe
alpha1-antitrypsin deficiency, and can assist in the recruitment of this
subpopulation of patients with emphysema for research studies.
14. What Is the Cost-Effectiveness of Strategies
for COPD Prevention and Treatment?
Despite substantial expenditures on medical care for
those with COPD, few studies have examined the cost-effectiveness of
different treatment modalities. It is likely that reductions in the
frequency and severity of acute exacerbations of COPD would be especially
cost-effective, because hospitalizations, primarily for exacerbations, account
for approximately two thirds of the direct costs of COPD care (Figure 1)
(79-81). Because those with advanced disease are more likely to be
hospitalized, therapeutic advances that produce even modest improvements in the
health of those with severe COPD may have a substantial economic impact.
Greater emphasis should be placed on studies of the cost-effectiveness of COPD
management approaches.
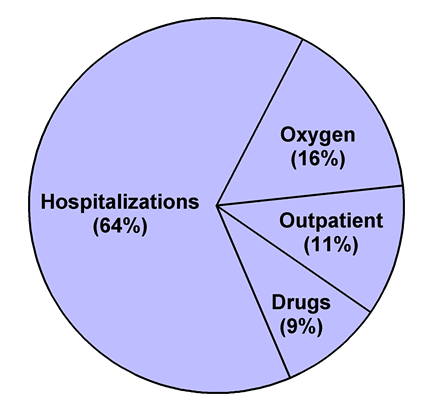
Figure 1. Allocation of expenditures for direct
costs of COPD care. Total expenditure for home oxygen therapy is from Reference
79 and may include some costs not due to COPD. Estimates of expenditures for
hospitalizations (inpatient plus emergency department), outpatient (clinic plus
physician office), and drugs are based on data from References 80 (prevalence
of COPD by hospitalizations) and 81 (costs per COPD patient for care related to
COPD).
RECOMMENDATIONS FOR FUTURE RESEARCH
The Working Group identified five major goals for
clinical research in COPD in the next several years. Each was rated as high
priority by a substantial majority of the participants. Collectively, these
five initiatives could provide a platform for addressing all of the important
clinical questions in COPD identified in the previous section.
Establish a Multicenter Clinical Research Network
to Perform Multiple, Short-term Clinical Trials of Treatments in Patients with
Moderate-to-Severe COPD
Controlled trials are needed to assess the efficacy of
and refine indications for various drugs currently used in COPD, including
beta2-agonists, anticholinergics, antibiotics, inhaled corticosteroids, and
mucus-altering drugs. In severe disease or exacerbations of disease, such
clinical trials can be completed in a relatively short period of time (e.g.,
1-2 years), but require access to a relatively large number of research
subjects, personnel experienced in clinical research, and appropriate
infrastructure for subject recruitment and data collection and analysis. An
efficient framework for such trials is a multicenter, multistudy Clinical
Research Network, established for the rapid design and implementation of
sequential or simultaneous clinical trials. A COPD Clinical Research Network is
needed to evaluate treatments currently used in individuals with COPD, with
emphasis on the management of acute exacerbations. This network could also
investigate subject stratification by phenotype, efficacy of novel agents,
treatment of coexisting heart disease, alternative markers of therapeutic
response (e.g., inspiratory capacity, quality of life), and interventions
related to muscle dysfunction, while obtaining longitudinal data on disease
progression in severe COPD.
Create a System for the Standardized Collection,
Processing, and Distribution of Lung Tissue Specimens and Associated Clinical
and Laboratory Data
A major need in COPD research is for correlation of
gene and protein expression in the lung with tissue structure, pulmonary
function, and disease status. The requisite tools are available, including a
wealth of antibodies for immunohistochemistry and advanced methods of molecular
histopathology that are capable of quantifying inflammatory cells, gene
expression, protein content, cellular phenotype, and microbial and viral
infections with exquisite sensitivity and high spatial resolution. Furthermore,
lung tissues are available for study from lobes excised for suspected cancer or
tissue removed in lung volume reduction surgery or lung transplantation.
Nonetheless, histopathologic research in COPD is impeded by the considerable
infrastructure and expense required to recruit and characterize lung tissue
donors, procure tissue samples, and process the specimens.
To enable molecular studies of COPD causation and
progression, the Working Group recommended that a Lung Tissue Resource be
established that would prepare and distribute to researchers collections of
tissue specimens obtained at surgery. This resource would make available
systematic collections of anonymized specimens, linked to extensive clinical
and laboratory data from the donor subjects. The program should collect
clinical data; perform specified clinical tests (including pulmonary function
testing and high resolution computed tomography); and harvest, process, and
distribute lung tissues. The program would complement recent initiatives of the
NHLBI in proteomics and genomics, adding important capabilities for studying
expression of specific genes in particular cell types and correlation of these
data with a detailed clinical and physiologic profile of the subject. Tissue
specimens linked to extensive phenotypic data would not only be invaluable for
research on disease pathogenesis, but would also be useful for studies of the
mechanisms of enhanced lung cancer risk in COPD, for identification and
validation of biomarkers, and for correlation of radiographic measures with
pathology.
Because the extent of disease often varies
substantially within a lobe, this program could also prepare tissue arrays from
single individuals by systematic sampling from central to peripheral regions of
a lobe. Such tissue arrays would be invaluable for correlating molecular
biological properties with local pathologic indices, including tissue structure
and inflammatory and microbiological measurements. In addition, the fact that
portions of each lung specimen can be provided to many different investigators
makes a centralized resource cost-efficient.
Develop Standards for the Classification and
Staging of COPD
Unlike many other diseases, only rudimentary standards
are available for describing the severity of COPD. A standardized method for
classifying patients with COPD is needed to allow comparisons among different
studies and to facilitate recognition of subpopulations that may differ in
responsiveness to specific therapeutic approaches. The system of classification
should include indicators of disease character, which might reflect differences
in pathogenesis, and of disease severity. Although spirometry is critical for
diagnosis of COPD, FEV1 may be of limited value in a classification scheme
designed to illuminate differences in disease mechanism among individuals. Any
system of patient classification in COPD will require periodic reassessment and
revision.
Characterize the Development and Progression of
COPD Using Measures and Biomarkers that Relate to Current Concepts of
Pathogenesis
The epidemiology and natural history of COPD are
primarily known in terms of FEV1 in male smokers, and there is little
description of the disease with regard to inflammatory status, biomarkers,
radiographic changes, genomics, proteomics, genetics of susceptibility,
socioeconomic factors, quality of life, and therapeutic responsiveness.
Improved understanding of COPD during the slow progression from preclinical to
moderate disease would aid the development of strategies for primary and
secondary prevention. A multicenter observational study that encompasses a wide
range of ages is needed to provide a more comprehensive description of the
disease. Although a longitudinal study has some advantages, a cross-sectional
design is considered to be more practical. Such a study could also be used to
validate biomarkers of COPD and to address hypotheses related to individual
susceptibility to cigarette smoke.
Evaluate Indications for Long-Term Oxygen Therapy
for Patients with COPD
There are discrepancies between scientific evidence,
physician practices, and insurance reimbursement policies with regard to
indications for long-term oxygen therapy. Although the life-prolonging
potential of oxygen may be great, oxygen is already a significant portion of
the total dollar cost of care for patients with COPD (Figure1). Hence, a strong
evidentiary basis is needed for guidelines for oxygen prescription. A
controlled clinical trial is especially needed to assess the benefit of
long-term oxygen therapy in individuals with moderate hypoxemia (e.g., PaO2
between 55 and 65 mm Hg). Such a trial might also address the issue of oxygen
supplementation for those who are normoxic at rest but desaturate with
ambulation, or who are normoxic by day but show nocturnal oxyhemoglobin
desaturation. Ancillary studies of noninvasive methods of mechanical
ventilatory support or of sleep disturbance and its management might also be
performed within the framework of a controlled trial of oxygen therapy.
Acknowledgment: Participants: A. Sonia Buist,
M.D., Portland, OR, and James D. Crapo, M.D., Denver, CO, Co-Chairs; Robert M.
Senior, M.D., St. Louis, MO, and Robert A. Wise, M.D., Baltimore, MD,
Discussion Leaders; and Nicholas R. Anthonisen, M.D., Ph.D., Winnipeg, MB,
Canada; Richard Casaburi, Ph.D., M.D., Torrance, CA; Gerard J. Criner, M.D.,
Philadelphia, PA; Thomas L. Croxton, Ph.D., M.D., Bethesda, MD; John V. Fahy,
M.D., San Francisco, CA; James E. Fish, Ph.D., Philadelphia, PA; Jonathan
Goldin, M.D., Ph.D., Los Angeles, CA; Edward P. Ingenito, M.D., Ph.D.,
Boston, MA; James P. Kiley, Ph.D., Bethesda, MD; Jenny Mao, M.D., Los Angeles,
CA; Fernando J. Martinez, M.D., Ann Arbor, MI; Dennis E. Niewoehner, M.D.,
Minneapolis, MN; Denis E. O’Donnell, M.D., Kingston, ON, Canada; Hector G.
Ortega, M.D., Sc.D., Bethesda, MD; Barbara Phillips, M.D., Lexington, KY; Sri
Ram, Ph.D., Bethesda, MD; Cynthia S. Rand, Ph.D., Baltimore, MD; Andrew L.
Ries, M.D., M.P.H., San Diego, CA; Gordon L. Snider, M.D., Boston, MA; Norbert
F. Voelkel, M.D., Denver, CO; Gail G. Weinmann, M.D., Bethesda, MD; and Roger
D. Yusen, M.D., M.P.H., St. Louis, MO.
References
- Owings MF, Lawrence L. Detailed diagnoses and
procedures, National Hospital Discharge Survey, 1997. Vital Health Stat
1999;13:1-157.
- Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek
KD. Deaths: final data for 1999. Natl Vital Stat Rep 2001;49:1-113.
- Nocturnal Oxygen Therapy Trial Group. Continuous
or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a
clinical trial. Ann Intern Med 1980;93:391-398.
- Croxton TL, Weinmann GG, Senior RM, Hoidal JR.
Future research directions in chronic obstructive pulmonary disease. Am J
Respir Crit Care Med 2002;165:838-844.
- American Thoracic Society. Standards for the
diagnosis and care of patients with chronic obstructive pulmonary disease. Am J
Respir Crit Care Med 1995;152:S77-S121.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR,
Hurd SS. Global strategy for the diagnosis, management, and prevention of
chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic
Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med
2001;163:1256-1276.
- Mannino DM, Gagnon RC, Petty TL, Lydick E.
Obstructive lung disease and low lung function in adults in the United States:
data from the National Health and Nutrition Examination Survey, 1988-1994. Arch
Intern Med 2000;160:1683-1689.
- Adams PF, Hendershot GE, Marano MA. Current
estimates from the National Health Interview Survey, 1996. Vital Health Stat
1999;10: 1-212.
- Schappert SM. Ambulatory care visits to physician
offices, hospital outpatient departments, and emergency departments: United
States, 1997. Vital Health Stat 1999;13:1-39.
- Collins JG. Prevalence of selected chronic
conditions: United States, 1990-1992. Vital Health Stat 1997;10:1-89.
- Sullivan SD, Ramsey SD, Lee TA. The economic
burden of COPD. Chest 2000;117:5S-9S.
- Karnath B. Smoking cessation. Am J Med
2002;112:399-405.
- Healton C, Messeri P, Reynolds J, Wolfe C, Stokes
C, Ross J, Flint K, Robb W, Farrelly M. Tobacco use among middle and high
school students-United States, 1999. MMWR Morb Mortal Wkly Rep 2000; 49:49-53.
- Centers for Disease Control and Prevention.
Incidence of initiation of cigarette smoking-United States, 1965-1996. MMWR
Morb Mortal Wkly Rep 1998;47:837-840.
- Anthonisen NR, Connett JE. Murray RP for Lung
Health Study Research Group. Smoking and lung function of Lung Health Study
participants after 11 years. Am J Respir Crit Care Med 2002;166:675-679.
- Medical Research Council Working Party. Long term
domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating
chronic bronchitis and emphysema. Lancet 1981;1:681-686.
- Mahler DA, Donohue JF, Barbee RA, Goldman MD,
Gross NJ, Wisniewski ME, Yancey SW, Zakes BA, Rickard KA, Anderson WH.
Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999;
115:957-965.
- COMBIVENT Inhalation Solution Study Group. Routine
nebulized ipratropium and albuterol together are better than either alone
in COPD. Chest 1997;112:1514-1521.
- Fink G, Kaye C, Sulkes J, Gabbay U, Spitzer SA.
Effect of theophylline on exercise performance in patients with severe chronic
obstructive pulmonary disease. Thorax 1994;49:332-334.
- Burge PS, Calverley PM, Jones PW, Spencer S,
Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of
fluticasone propionate in patients with moderate to severe chronic obstructive
pulmonary disease: the ISOLDE trial. BMJ 2000;320:1297-1303.
- Lung Health Study Research Group. Effect of
inhaled triamcinolone on the decline in pulmonary function in chronic
obstructive pulmonary disease. N Engl J Med 2000;343:1902-1909.
- Niewoehner DE, Erbland ML, Deupree RH, Collins D,
Gross NJ, Light RW, Anderson P, Morgan NA. Effect of systemic glucocorticoids
on exacerbations of chronic obstructive pulmonary disease: Department of
Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340: 1941-1947.
- Thompson WH, Nielson CP, Carvalho P, Charan NB,
Crowley JJ. Con-trolled trial of oral prednisone in outpatients with acute COPD
exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
- Schols AM, Wesseling G, Kester AD, de Vries G,
Mostert R, Slangen J, Wouters EF. Dose dependent increased mortality risk in
COPD patients treated with oral glucocorticoids. Eur Respir J 2001;17:337-342.
- Sethi S, Murphy TF. Bacterial infection in chronic
obstructive pulmonary disease in 2000: a state-of-the-art review. Clin
Microbiol Rev 2001;14: 336-363.
- Saint S, Bent S, Vittinghoff E, Grady D.
Antibiotics in chronic obstructive pulmonary disease exacerbations. A
meta-analysis. JAMA 1995;273: 957-960.
- Bach PB, Brown C, Gelfand SE, McCrory DC.
Management of acute exacerbations of chronic obstructive pulmonary disease: a
summary and appraisal of published evidence. Ann Intern Med 2001;134:600-620.
- Poole PJ, Black PN. Oral mucolytic drugs for
exacerbations of chronic obstructive pulmonary disease: systematic review. BMJ
2001;322: 1271-1274.
- American College of Chest Physicians and American
Association of Cardiovascular and Pulmonary Rehabilitation Guidelines Panel.
Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based guide-lines.
Chest 1997;112:1363-1396.
- American Thoracic Society. Pulmonary
rehabilitation—1999. Am J Respir Crit Care Med 1999;159:1666-1682.
- Schwaiblmair M, Reichenspurner H, Muller C,
Briegel J, Furst H, Groh J, Reichart B, Vogelmeier C. Cardiopulmonary exercise
testing before and after lung and heart-lung transplantation. Am J Respir Crit
Care Med 1999;159:1277-1283.
- Gross CR, Savik K, Bolman RM III, Hertz MI.
Long-term health status and quality of life outcomes of lung transplant
recipients. Chest 1995; 108:1587-1593.
- Hosenpud JD, Bennett LE, Keck BM, Edwards EB,
Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for
end-stage lung disease. Lancet 1998;351:24-27.
- De Meester J, Smits JM, Persijn GG, Haverich A.
Listing for lung trans-plantation: life expectancy and transplant effect,
stratified by type of end-stage lung disease, the Eurotransplant experience. J
Heart Lung Transplant 2001;20:518-524.
- Charman SC, Sharples LD, McNeil KD, Wallwork J.
Assessment of survival benefit after lung transplantation by patient diagnosis.
J Heart Lung Transplant 2002;21:226-232.
- Flaherty KR, Kazerooni EA, Curtis JL, Iannettoni
M, Lange L, Schork MA, Martinez FJ. Short-term and long-term outcomes after
bilateral lung volume reduction surgery: prediction by quantitative CT. Chest
2001;119:1337-1346.
- National Emphysema Treatment Trial Research Group.
Rationale and design of the National Emphysema Treatment Trial (NETT): a
prospective randomized trial of lung volume reduction surgery. J Thorac
Cardiovasc Surg 1999;118:518-528.
- Eriksson S. A 30-year perspective on alpha
1-antitrypsin deficiency. Chest 1996;110:237S-242S.
- Shapiro SD, Senior RM. Matrix metalloproteinases:
matrix degradation and more. Am J Respir Cell Mol Biol 1999;20:1100-1102.
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD.
Requirement for macrophage elastase for cigarette smoke-induced emphysema in
mice. Science 1997;277:2002-2004.
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek
A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, et al. Fibulin-5/DANCE
is essential for elastogenesis in vivo. Nature 2002;415:171-175.
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T,
Yanagisawa M, Richard-son JA, Olson EN. Fibulin-5 is an elastin-binding protein
essential for elastic fibre development in vivo. Nature 2002;415:168-171.
- Barnes PJ. Mechanisms in COPD: differences from
asthma. Chest 2000; 117:10S-14S.
- Hogg JC. Role of latent viral infections in
chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med
2001;164:S71-S75.
- MacNee W. Oxidants/antioxidants and COPD. Chest
2000;117:303S-317S.
- Fu X, Kassim SY, Parks WC, Heinecke JW.
Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin
(MMP-7): a mechanism for matrix metalloproteinase activation and
atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem
2001;276:41279-41287.
- Okamoto K, Tanaka H, Ogawa H, Makino Y, Eguchi H,
Hayashi S, Yoshikawa N, Poellinger L, Umesono K, Makino I.
Redox-dependent regulation of nuclear import of the glucocorticoid receptor. J
Biol Chem 1999;274:10363-10371.
- Kharitonov SA, Barnes PJ. Exhaled markers of
pulmonary disease. Am J Respir Crit Care Med 2001;163:1693-1722.
- Smit HA, Grievink L, Tabak C. Dietary influences
on chronic obstructive lung disease and asthma: a review of the epidemiological
evidence. Proc Nutr Soc 1999;58:309-319.
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le
Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF
receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:
1311-1319.
- Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores
SC, Voelkel NF. Endothelial cell death and decreased expression of vascular
endothelial growth factor and vascular endothelial growth factor receptor
2 in emphysema. Am J Respir Crit Care Med 2001;163:737-744.
- Mao JT, Goldin JG, Dermand J, Ibrahim G, Brown MS,
Emerick A, McNitt-Gray MF, Gjertson DW, Estrada F, Tashkin DP, et al. A pilot
study of all-trans-retinoic acid for the treatment of human emphysema. Am J
Respir Crit Care Med 2002;165:718-723.
- Ferguson GT, Enright PL, Buist AS, Higgins MW.
Office spirometry for lung health assessment in adults: a consensus statement
from the National Lung Health Education Program. Chest 2000;117:1146-1161.
- Risser NL, Belcher DW. Adding spirometry, carbon
monoxide, and pulmonary symptom results to smoking cessation counseling:
a randomized trial. J Gen Intern Med 1990;5:16-22.
- Sippel JM, Osborne ML, Bjornson W, Goldberg B,
Buist AS. Smoking cessation in primary care clinics. J Gen Intern Med
1999;14:670-676.
- Jimenez-Ruiz CA, Masa F, Miravitlles M, Gabriel R,
Viejo JL, Villasante C, Sobradillo V. Smoking characteristics: differences in
attitudes and dependence between healthy smokers and smokers with COPD. Chest
2001;119:1365-1370.
- Silverman EK. Genetic epidemiology of COPD. Chest
2002;121:1S-6S.
- Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis
CR, Hawthorne VM. Impaired lung function and mortality risk in men and women:
findings from the Renfrew and Paisley prospective population study. BMJ
1996;313:711-715.
- MacNee W. Oxidative stress and lung inflammation
in airways disease. Eur J Pharmacol 2001;429:195-207.
- Ross JS, Stagliano NE, Donovan MJ, Breitbart RE,
Ginsburg GS. Atherosclerosis and cancer: common molecular pathways of
disease development and progression. Ann NY Acad Sci 2001;947:271-292.
- O'Donnell DE, Lam M, Webb KA. Spirometric
correlates of improvement in exercise performance after anticholinergic
therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med
1999;160: 542-549.
- Hatipoglu US, Laghi F, Tobin MJ. Does inhaled
albuterol improve diaphragmatic contractility in patients with chronic
obstructive pulmonary disease? Am J Respir Crit Care Med 1999;160:1916-1921.
- Jones PW. Health status measurement in chronic
obstructive pulmonary disease. Thorax 2001;56:880-887.
- Dirksen A, Dijkman JH, Madsen F, Stoel B,
Hutchison DC, Ulrik CS, Skovgaard LT, Kok-Jensen A, Rudolphus A, Seersholm N,
et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation
therapy. Am J Respir Crit Care Med 1999;160:1468-1472.
- Stoller JK. Clinical practice: acute exacerbations
of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
- Connors AF Jr, Dawson NV, Thomas C, Harrell FE Jr,
Desbiens N, Fulkerson WJ, Kussin P, Bellamy P, Goldman L, Knaus WA. Out-comes
following acute exacerbation of severe chronic obstructive lung disease: the
SUPPORT investigators (Study to Understand Prognoses and Preferences for
Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959-967.
- Petty TL, Casaburi R. Recommendations of the Fifth
Oxygen Consensus Conference. Writing and Organizing Committees. Respir Care
2000;45: 957-961.
- Carter R, Nicotra B, Huber G. Differing effects of
airway obstruction on physical work capacity and ventilation in men and women
with COPD. Chest 1994;106:1730-1739.
- Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in
chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;133:14-20.
- O'Donnell DE, Revill SM, Webb KA. Dynamic
hyperinflation and exer- NHLBI Workshop Summary cise intolerance in chronic
obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:770-777.
- American Thoracic Society and European Respiratory
Society. Skeletal muscle dysfunction in chronic obstructive pulmonary disease:
a statement of the American Thoracic Society and European Respiratory Society.
Am J Respir Crit Care Med 1999;159:S1-S40.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal
TP. Prognostic value of nutritional status in chronic obstructive pulmonary
disease. Am J Respir Crit Care Med 1999;160:1856-1861.
- Ferreira IM, Brooks D, Lacasse Y, Goldstein RS.
Nutritional support for individuals with COPD: a meta-analysis. Chest
2000;117:672-678.
- McNicholas WT. Impact of sleep in COPD. Chest
2000;117:48S-53S.
- Murciano D, Armengaud MH, Cramer PH, Neveux E,
L'Heritier C, Pariente R, Aubier M. Acute effects of zolpidem, triazolam and
flunitrazepam on arterial blood gases and control of breathing in severe COPD.
Eur Respir J 1993;6:625-629. 1149
- Douglas NJ, Flenley DC. Breathing during sleep in
patients with obstructive lung disease. Am Rev Respir Dis
1990;141:1055-1070.
- Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz
HM. Effectiveness of beta-blocker therapy after acute myocardial
infarction in elderly patients with chronic obstructive pulmonary disease
or asthma. J Am Coll Cardiol 2001;37:1950-1956.
- Edwards JG, Duthie DJ, Waller DA. Lobar volume
reduction surgery: a method of increasing the lung cancer resection rate in
patients with emphysema. Thorax 2001;56:791-795.
- O'Donohue WJ Jr, Plummer AL. Magnitude of usage
and cost of home oxygen therapy in the United States. Chest 1995;107:301-302.
- Stang P, Lydick E, Silberman C, Kempel A, Keating
ET. The prevalence of COPD: using smoking rates to estimate disease frequency
in the general population. Chest 2000;117:354S-359S. 81. Strassels SA, Smith
DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States.
Chest 2001;119:344-352.
Correspondence and requests for reprints should be
addressed to:
Thomas Croxton, Ph.D., M.D.
Division of Lung Diseases
National Heart, Lung, and Blood Institute
6701 Rockledge Drive, Room
10208
Bethesda, MD 20892-7952.
E-mail:
croxtont@nhlbi.nih.gov
|
