|
CFSAN/Office of Nutrition, Labeling, and Dietary Supplements
April 2008
Answer: There are two ways to label packages and containers:
21 CFR 101.2, 21 CFR 101.3, 21 CFR 101.4, 21 CFR 101.9, and 21 CFR 101.105,
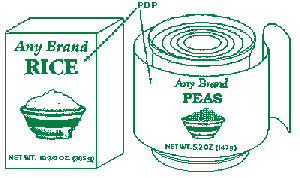 What are the PDP and the alternate PDP?
What are the PDP and the alternate PDP?
Answer: The PDP, is that portion of the package label that is most likely to be seen by the consumer at the time of purchase. Many containers are designed with two or more different surfaces that are suitable for display as the PDP. These are alternate PDPs.
 What label statements must appear on the PDP?
What label statements must appear on the PDP?
Answer: Place the statement of identity, or name of the food, and the net quantity statement, or amount of product, on the PDP and on the alternate PDP. The required type size and prominence are discussed in Chapters IV and V of this guidance and 21 CFR 101.3(a) and 21 CFR 101.105(a)
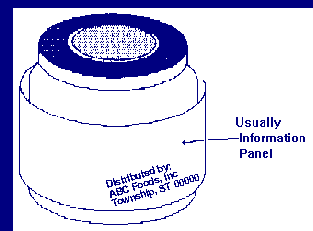
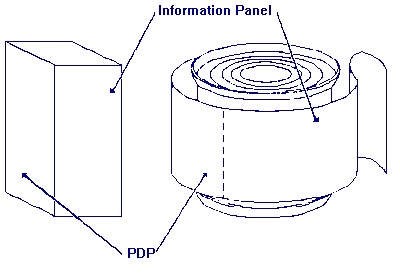 Which label panel is the information panel?
Which label panel is the information panel?
Answer: The information panel is the label panel immediately to the right of the PDP, as displayed to the consumer. If this panel is not usable, due to package design and construction, (e.g., folded flaps), then the information panel is the next label panel immediately to the right.
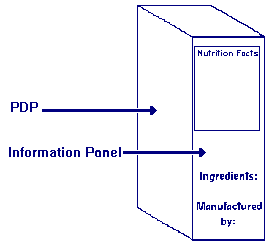 What is information panel labeling?
What is information panel labeling?
Answer: The phrase "information panel labeling" refers to the label statements that are generally required to be placed together, without any intervening material, on the information panel, if such labeling does not appear on the PDP. These label statements include the name and address of the manufacturer, packer or distributor, the ingredient list, and nutrition labeling.
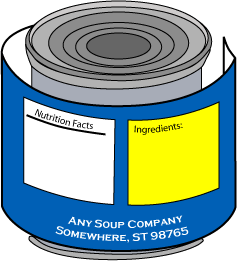 What type size, prominence and conspicuousness is required?
What type size, prominence and conspicuousness is required?
Answer: For information panel labeling, use a print or type size that is prominent, conspicuous and easy to read. Use letters that are at least one-sixteenth (1/16) inch in height based on the lower case letter "o". The letters must not be more than three times as high as they are wide, and the lettering must contrast sufficiently with the background so as to be easy to read. Do not crowd required labeling with artwork or non-required labeling.
Smaller type sizes may be used for information panel labeling on very small food packages as discussed in 21 CFR 101.2(c).
Different type sizes are specified for the Nutrition Facts Label.
The type size requirements for the statement of identity and the net quantity statement are discussed in section V of this guidance.
21 CFR 101.2(c) and 21 CFR 101.9(d)(1)(iii)
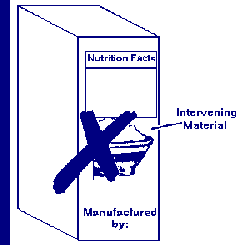 What is the prohibition against intervening material?
What is the prohibition against intervening material?
Answer: Nonessential, intervening material is not permitted to be placed between the required labeling on the information panel (e.g., the UPC bar code is not required labeling).
 What name and address must be listed on the label?
What name and address must be listed on the label?Answer: Food labels must list: