
|
 |
Hospital eTool
Laboratory Module
Click on the area for more specific information.

|
Common Safety and health issues:
Bloodborne pathogens are pathogenic microorganisms present in human blood that can cause disease in humans. These pathogens include,
but are not limited to, Hepatitis B Virus (HBV),
Hepatitis C Virus
(HCV), and
Human Immunodeficiency Virus (HIV). Hepatitis B can
survive in dried blood specimens for several days.
Definitions for bloodborne pathogens, Other Potentially Infectious Materials (OPIM), and occupational exposure are found in
[29
CFR 1910.1030(b)].
|
Potential Hazard
Exposure of laboratory employees to bloodborne pathogens while handling contaminated lab samples such as blood
or other body fluids (i.e., cerebrospinal fluid, and semen).
Possible Solutions
Compliance with the Bloodborne Pathogens Standard. For example:
- Wear appropriate PPE as required by the Bloodborne Pathogens Standard 29
CFR 1910.1030(d)(3)(i)
if blood or Other Potentially Infectious Materials (OPIM) exposure is anticipated. The type and amount of PPE depends on the
anticipated exposure.
- Gloves must be worn when hand contact with blood, mucous membranes, OPIM, or non-intact skin is anticipated, or when handling
contaminated items or surfaces [29
CFR 1910.1030(d)(ix)].
- Provide effective engineering and work practice controls to help remove or isolate exposures to blood and bloodborne pathogens
(29
CFR 1910.1030(c)(1)(iv), CPL 02-02-069 [CPL 2-2.69]).
- Employers must offer the Hepatitis B vaccination under the supervision of a licensed physician at no cost to all employees who have
occupational exposure to blood or OPIM [29
CFR 1910.1030(2)].
 For additional information see Healthcare Wide Hazards -
Bloodborne Pathogens.
For additional information see Healthcare Wide Hazards -
Bloodborne Pathogens.
Additional Bloodborne Pathogen Standard requirements apply for HIV and HBV Research Laboratories.
They do not apply to clinical or diagnostic laboratories engaged solely in the analysis of blood, tissues, or organs
[29
CFR 1910.1030(e)(i)].
Some of these additional requirements include:
-
Waste materials:
- All regulated waste shall either be incinerated or decontaminated by a method such as autoclaving known to effectively destroy
bloodborne pathogens
[29 CFR 1910.1030(e)(2)(i)].
- Contaminated materials that are to be decontaminated at a site away from the work area shall be placed in a durable, leak proof,
labeled or color-coded container that is closed before being removed from the work area
[29 CFR 1910.1030(e)(2)(ii)(B)].
-
Access:
- Laboratory doors shall be kept closed when work involving HIV or HBV is in progress
[29 CFR 1910.1030(e)(2)(ii)(A)].
- Access to the work area shall be limited to authorized persons. Written policies and procedures shall be established whereby only
persons who have been advised of the potential biohazard, who meet any specific entry requirements, and who comply with all entry and
exit procedures shall be allowed to enter the work areas and animal rooms
[29 CFR 1910.1030(e)(2)(ii)(C)].
- Access doors to the work area or containment module shall be self-closing
[29 CFR 1910.1030(e)(4)(iv)].
- The work areas shall be separated from areas that are open to unrestricted traffic flow within the building. Passage through two sets
of doors shall be the basic requirement for entry into the work area from access corridors or other contiguous areas. Physical
separation of the high-containment work area from access corridors or other areas or activities may also be provided by a
double-doored
clothes-change room (showers may be included), airlock, or other access facility that requires passing through two sets of doors
before entering the work area
[29 CFR 1910.1030(e)(4)(i)].
- The surfaces of doors, walls, floors and ceilings in the work area shall be water resistant so that they can be easily cleaned.
Penetrations in these surfaces shall be sealed or capable of being sealed to facilitate decontamination
[29 CFR 1910.1030(e)(4)(ii)].
- Labels:
- When other potentially infectious materials or infected animals are present in the work area or containment module, a hazard warning
sign incorporating the universal biohazard symbol shall be posted on all access doors. The hazard warning sign shall comply with
paragraph
29 CFR 1910.1030(g)(1)(ii) of this standard
[29 CFR 1910.1030(e)(2)(ii)(D)].
- Engineering Controls and Work Practice:
- All activities involving other potentially infectious materials shall be conducted in biological safety cabinets or other physical-containment
devices within the containment module. No work with these other potentially infectious materials shall be conducted on the open bench
[29 CFR 1910.1030(e)(2)(ii)(E)].
- Certified biological safety cabinets (Class I, II, or III) or other appropriate combinations of personal protection or physical
containment devices, such as special protective clothing, respirators, centrifuge safety cups, sealed centrifuge rotors, and containment
caging for animals, shall be used for all activities with other potentially infectious materials that pose a threat of exposure to
droplets, splashes, spills, or aerosols
[29 CFR 1910.1030(e)(2)(iii)(A)].
- Each work area shall contain a sink for washing hands and a readily available eye wash facility. The sink shall be foot, elbow, or
automatically operated and shall be located near the exit door of the work area
[29 CFR 1910.1030(e)(4)(iii)].
- Each laboratory shall contain a facility for hand washing and an eye wash facility which is readily available within the work area
[29 CFR 1910.1030(e)(3)(i)].
Additional Information:
- Centers for Disease Control and
Prevention (CDC), National Institutes of Health (NIH). Biosafety Manual.
4th ed. Washington, DC: US Government Printing Office, 1999.
|
In 1997 OSHA estimated that more than 5.3 million workers in more than 100,000 hospitals, homeless
shelters, long-term care facilities for the elderly, detention facilities, certain laboratories and other work settings have a
high risk of TB infection. In the United States, 13 million adults are presently believed to be infected with TB, and 22,813 have
active cases that were reported in the U.S. in 1995. The CDC considers workers in medical laboratories that handle M. tuberculosis
to be at high risk for occupational transmission of TB. The potential of contracting TB among persons who work with TB in the lab
is three to five times greater than among lab personnel that do not work with TB bacterium.
|
Potential Hazard
Exposure of laboratory employees to TB from working with specimens (e.g., acid fast bacilli smears), that may
contain tuberculosis. Other fluids that may be potential sources of TB are sputum, cerebrospinal fluid urine, and fluids collected
from gastric or bronchial lavage.
Possible Solutions
-
All cultures or specimens suspected of containing TB bacilli must be manipulated in settings where specific engineering controls,
administrative procedures, and appropriate personal work practices ensure containment of the organism and protection of the workers
These practices should address issues including:
- Biosafety Level: In order for a laboratory to handle TB sputum and TB materials, the laboratory must operate at a
biosafety level
of 2+ or 3.
-
Controlled access, anterooms, sealed windows, directional airflow, preventing recirculation of laboratory exhaust air, filtration of
exhaust air before discharge to the outside, and thimble exhaust connections for biological safety.
- The use of biological safety cabinets whenever working with infectious materials that have a chance of aerosolizing. Processes that
can expose employees to aerosolized materials include:
- Pouring liquid cultures
- Using fixed-volume automatic pipettors
- Mixing liquid cultures with a pipette
- Preparing specimens and culture smears
- Dropping and spilling tubes containing suspensions of bacilli
 For additional information, see Healthcare Wide Hazards -
Tuberculosis.
For additional information, see Healthcare Wide Hazards -
Tuberculosis.
Additional Information:
|
OSHA Laboratory Standard
|
|
The Laboratory Standard applies to all hazardous chemicals meeting the definition of
"laboratory use" and having a potential for employee exposure. |
|
Potential Hazard
Staff exposure to hazardous laboratory chemicals.
Possible Solutions
- Follow OSHA Standards including: 29
CFR 1910.1450,
Occupational exposure to hazardous chemicals in laboratories, if applicable.
- Appendix A, National Research Council Recommendations Concerning
Chemical Hygiene in Laboratories (Non-Mandatory)
- Appendix B, References (Non-Mandatory)
- The laboratory standard 29 CFR 1910.1450 may apply to hospital laboratories:
- "Laboratory" means a facility where the "laboratory use of
hazardous chemicals" occurs. It is a workplace where
relatively small quantities of hazardous chemicals are used on a non-production basis.
- "Laboratory use of hazardous chemicals" means handling or use of such chemicals
in which all of the following conditions are met:
- (i) Chemical manipulations are carried out on a "laboratory scale;"
- (ii) Multiple chemical procedures or chemicals are used;
- (iii) The procedures involved are not part of a production
process, nor in any way simulate a production process; and
- (iv) "Protective laboratory practices and
equipment" are available and in common use to minimize
the potential for employee exposure to hazardous chemicals.
- Any hazardous chemical use which does not meet this definition is regulated under other standards. This
includes other hazardous chemical use within a laboratory. For instance:
- Chemicals used in building maintenance of a laboratory are not covered under the laboratory standard.
- The production of a chemical for commercial sale, even in small quantities is not covered.
- Quality control testing of a product is not covered under the laboratory standard.
- If the laboratory standard 29 CFR 1910.1450 applies, employers must develop a Chemical Hygiene Plan.
- The Chemical Hygiene Plan is the laboratory's program which addresses all aspects of the Laboratory Standard. The employer
is required to develop and carry out the provisions of a written Chemical Hygiene Plan. Until a written plan is fully implemented
the laboratory is regulated under the general industry standards.
- There are numerous chemical hygiene plans available on the Internet. Most of these are from colleges, universities, and
governmental facilities. Examples include:
- The following OSHA Standard Interpretations on the Laboratory Standard may provide additional information:
Additional Information:
|
The employer shall assure that no employee is exposed to an airborne concentration of formaldehyde which
exceeds 0.75 parts formaldehyde per million parts of air (0.75 ppm) as an 8-hour TWA
[29
CFR 1910.1048(c)(1)].
|
Potential Hazards
Employee exposure to Formaldehyde. Formaldehyde is used as a fixative and is commonly found in most
laboratories and the morgue (29 CFR
1910.1048).
Health Effects:
|
- Acute: Eye and respiratory irritation can result from exposure to the liquid and vapor forms. Severe abdominal pains, nausea,
vomiting and possible loss of consciousness could occur, if ingested in large amounts.
- Chronic: High concentration of vapor inhaled for long periods can cause laryngitis, bronchitis or bronchial pneumonia. Prolonged
exposure may cause conjunctivitis. Nasal tumors have been reported in animals. Formaldehyde is a suspected carcinogen.
|
Possible Solutions
|
|
According to the 29
CFR 1910.148 Formaldehyde Standard:
|

|
- If there is any possibility that an employee's eyes may be splashed with solutions containing 0.1 percent or greater formaldehyde, the
employer shall provide acceptable eyewash facilities within the immediate work area for emergency use
[29
CFR 1910.1048(i)(3)].
|
 For additional information, see Healthcare Wide Hazards -
PPE.
For additional information, see Healthcare Wide Hazards -
PPE.
|
|
|
Toluene,
Xylene, or Acryl Amide Exposure
|
Potential Hazard
Employee exposure to hazardous chemicals such as Toluene, Xylene or Acryl amide. Toluene and Xylene are solvents used to fix tissue
specimens and rinse stains. They are primarily found in the histology, hematology, microbiology, and cytology laboratories
(29 CFR
1910.1000 Subpart Z). Acryl amide a resin, usually found in research labs, is used to make gels for biochemical separations.
Health Effects:
-
Toluene and Xylene Exposure:
Acute: Eye and respiratory irritation can result form exposure to the liquid and vapor forms. Severe
abdominal pains, nausea, vomiting and possible loss of consciousness could occur, if ingested in large amounts.
Chronic: High concentration of vapor inhaled for long periods can cause laryngitis, bronchitis or bronchial
pneumonia. Prolonged exposure may cause conjunctivitis. Nasal tumors have been reported in animals.
- Acryl Amide Exposure:
Acute: Eye and skin irritation.
Chronic: Central nervous system disorders, i.e.,
polyneuropathy. Acryl amide is a suspected carcinogen, and mutagen.
Possible Solutions
- OSHA requires that employers implement a written program that meets the requirements of the
Hazard Communication Standard (HCS) to provide for worker training, warning labels, and access to Material Safety Data Sheets
(MSDSs).
- Occupational Exposure to Hazardous Chemicals in Laboratories. 29
CFR 1910.1450
 For additional information, see Healthcare Wide Hazards -
Hazardous Chemicals.
For additional information, see Healthcare Wide Hazards -
Hazardous Chemicals.
Additional Information:
|
|
Needle Stick or Sharps Injuries
|
Potential Hazard
Employee exposure to bloodborne pathogens from needlestick injuries or cuts from sharp objects when working with specimens, centrifuge
tubes or overfilled sharps containers.
Possible Solutions

|
- Use engineering controls (e.g., safer needle devices), and work practice controls (e.g., altering the way a task is performed to
reduce chance of injury such as prohibiting recapping of needles by a two-handed technique), to eliminate or minimize exposure to
bloodborne pathogens.
-
OSHA, FDA and NIOSH warn health care workers about the hazards from breakage of glass capillary tubes and recommend using non-glass
capillary tubes. For additional information, see Health Care Wide Hazards -
Needlesticks.
- Do not allow sharps containers to overfill, but replace routinely [29
CFR 1910.1030(d)(4)(iii)(A)(2)(iii)].
- Additional Bloodborne Pathogen Standard requirements that apply to HIV and HBV Research Laboratories include:
- Hypodermic needles and syringes shall be used only for parenteral injection and aspiration of fluids from laboratory animals and
diaphragm bottles. Only needle-locking syringes or disposable syringe-needle units (i.e., the needle is integral to the syringe)
shall be used for the injection or aspiration of other potentially infectious materials. Extreme caution shall be used when handling
needles and syringes. A needle shall not be bent, sheared, replaced in the sheath or guard, or removed from the syringe following use.
The needle and syringe shall be promptly placed in a puncture-resistant container and autoclaved or decontaminated before reuse or disposal
[29
CFR 1910.1030(e)(2)(ii)(J)].
|
 For additional information, see Healthcare Wide Hazards -
Bloodborne Pathogens.
For additional information, see Healthcare Wide Hazards -
Bloodborne Pathogens.
|
|
Work Practices and Behaviors
|
Potential Hazards
Poor work practices and behaviors can cause worker exposure to hazardous chemicals and diseases, (e.g., scratching nose or chewing pencils or pens
when working with hazardous samples).
Possible Solutions
- Careful monitoring of work behaviors and habits to prevent exposures.
- Some employees routinely double glove so that the outer glove can be removed if the employee needs to scratch or answer a phone and then replaced with
a new glove when ready to go back to work.
- The Bloodborne Pathogens Standard requirements include:
|
- No Mouth pipetting/suctioning of blood or other potentially infectious materials is allowed
[29
CFR 1910.1030(d)(2)(xii)].
- No Eating, drinking, smoking, applying cosmetics or lip balm, or handling contact lenses is allowed in work areas where there is a
reasonable likelihood of occupational exposure to bloodborne pathogens [29
CFR 1910.1030(d)(2)(ix)].
|
|

|
- No storage of food or drink in refrigerators, freezers, shelves, cabinets or on countertops or bench tops where blood or other
potentially infectious materials are present [29
CFR 1910.1030(d)(2)(x)].
|

|
Additional Information:
|
|
Engineering Controls
|
Potential Hazards
Staff exposure to infectious materials/organisms.
Possible Solutions
Use engineering controls such as:

|
- Splatter guards: to prevent splashing from reaching employee, (e.g., plexiglass barriers).
|
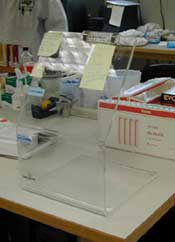
|

|
- Sensor-controlled "automatic sinks" or foot, knee, or elbow controls are available on sinks to operate
hand-washing facilities without using hands.
|

|
|
- Centrifuge tubes with caps.
|
|

|
- Biological Safety Cabinets
- Check daily for proper air exchange and air flow.
- Keep maintenance records for ventilation systems and other equipment.
|

|
- Maintenance records for laboratory hoods and other equipment.
|
|
|
Morgue
|
Potential Hazard
Employee exposure to infectious diseases and agents, (e.g.,
staph, strep, TB, HIV, HBV), and chemicals such as
Formaldehyde from contact with cadavers.
Possible Solutions
-
Use
Universal Precautions as required by the Bloodborne Pathogens Standard
29 CFR 1910.1030.
- Wear appropriate PPE, addressed in [29
CFR 1910.132(a)],
(e.g., gloves, goggles, gowns).
- The Bloodborne Pathogens Standard requires PPE if blood exposure is anticipated
[29
CFR 1910.1030(d)(3)(i)],
and additional PPE may be required during autopsies or orthopedic surgery such as:
- Surgical caps or hoods and/or shoe covers or boots shall be worn in instances when gross contamination can reasonably be anticipated
[29
CFR 1910.1030(d)(3)(xii)].
-
Engineering Controls:
- Provide appropriate ventilation systems (e.g., downdraft tables that capture the air around the cadaver).
- Place local vacuum systems for power saws in the morgue. Shields should be in place when significant splash
hazards are anticipated.
- Use splatter guards (e.g., plexiglass), to prevent splashes from reaching employee.
 For additional information, see Healthcare Wide Hazards -
PPE,
Tuberculosis, and
MRO-MRSA infections.
For additional information, see Healthcare Wide Hazards -
PPE,
Tuberculosis, and
MRO-MRSA infections.
|

|
Latex Allergy
|
|
Potential Hazard
Exposure of employees to latex allergy from wearing latex gloves.
Possible Solutions
Employers must provide appropriate gloves when exposure to blood or other potentially infectious materials (OPIM)
exists [29
CFR 1910.1030
Bloodborne Pathogens Standard].
- Alternatives shall be readily accessible to those employees who are allergic to the gloves normally
provided [29
CFR 1910.1030(d)(3)(iii)].
|

Nitrile Non-latex gloves
|
- Among the alternatives are synthetic, low-protein, and powder-free gloves. Powder-free gloves may reduce systemic allergic responses.
|
 For additional information, see Healthcare Wide Hazards -
Latex Allergy.
For additional information, see Healthcare Wide Hazards -
Latex Allergy.
|
 Slips/Trips/Falls
Slips/Trips/Falls
|
Potential Hazard
Staff exposure to trips and falls if fluids or samples fall to the floor.
Possible Solutions
-
Walking/Working Surfaces Standard requires [29
CFR 1910.22(a)(1)]:
All places of employment shall be kept clean and orderly and in a sanitary condition.
- Good work practice recommends rapid cleanup of spills.
 For additional information, see Healthcare Wide Hazards -
Slips/Trips/Falls.
For additional information, see Healthcare Wide Hazards -
Slips/Trips/Falls.
|
|
Ergonomics
|
Potential Hazard
-
Employee exposure to static postures from long periods of sitting or standing, or repetitive motions if sorting samples.
Possible Solutions
|
-
Install automated tract delivery systems for specimen processing to minimize employee reaching and repetitive motions.
|

|

|
-
Provide supportive comfortable chairs that include foot rests.
|

|
|
-
Rotate tasks or minimize the amount of time spent at these tasks.
|
 For additional information, see Healthcare Wide Hazards -
Ergonomics.
For additional information, see Healthcare Wide Hazards -
Ergonomics.
|
|
|

