Direct Observation of Hydrogen Adsorption Sites and Nano-Cage Formation
in Metal-Organic Frameworks (MOF) [T. Yildirim and M. R. Hartman, Cond-mat/0507220 ]
 In this study, we have used neutron scattering techniques to visualize the available adsorption sites in MOF materials directly. Fourier difference analysis of the neutron diffraction data clearly show that the molecules are packed in a fashion similar to the way apples or oranges fill a bowl. Surprisingly, we found that the MOF host lattice has the right-space available to hold many hydrogen molecules, up to ~10 wt. % at low temperatures. The metal-oxide cluster is responsible for most of the adsorption while the organic linker plays only a secondary role. These results suggest that by using different organic linkers, which make the hydrogen desorption difficult by narrowing the channels connecting the network of nano-pores in MOF, one may be able to optimize these materials for practical hydrogen storage at ambient conditions.
Equally important, we show that at high-concentration loadings hydrogen molecules form unique 3D networks of hydrogen nano-clusters with intermolecular distances as small as 3.0 Å, which in pure solid hydrogen can only be reached at high pressure. These findings suggest that MOF materials can also be used as templates to create artificial, interlinked hydrogen nano-cages. Such materials could exhibit very unexpected electronic properties due to these small intermolecular distances, such as metallic or superconducting behavior.
SEE FULL STORY..
In this study, we have used neutron scattering techniques to visualize the available adsorption sites in MOF materials directly. Fourier difference analysis of the neutron diffraction data clearly show that the molecules are packed in a fashion similar to the way apples or oranges fill a bowl. Surprisingly, we found that the MOF host lattice has the right-space available to hold many hydrogen molecules, up to ~10 wt. % at low temperatures. The metal-oxide cluster is responsible for most of the adsorption while the organic linker plays only a secondary role. These results suggest that by using different organic linkers, which make the hydrogen desorption difficult by narrowing the channels connecting the network of nano-pores in MOF, one may be able to optimize these materials for practical hydrogen storage at ambient conditions.
Equally important, we show that at high-concentration loadings hydrogen molecules form unique 3D networks of hydrogen nano-clusters with intermolecular distances as small as 3.0 Å, which in pure solid hydrogen can only be reached at high pressure. These findings suggest that MOF materials can also be used as templates to create artificial, interlinked hydrogen nano-cages. Such materials could exhibit very unexpected electronic properties due to these small intermolecular distances, such as metallic or superconducting behavior.
SEE FULL STORY..
Calculations suggest transition metal-decorated nanostuctures as potential high-capacity hydrogen storage medium [Phys. Rev. Lett. 94, 175501 (2005)]
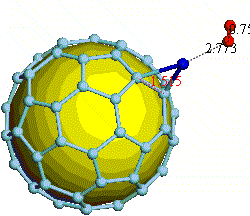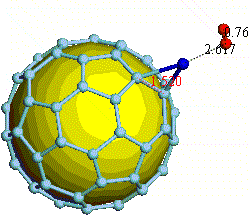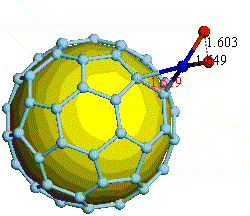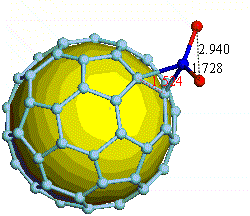
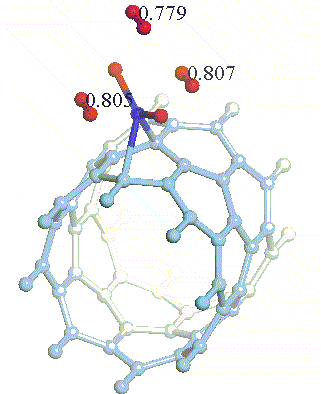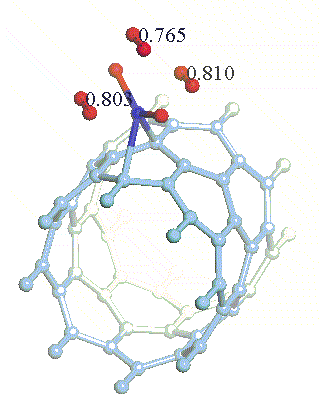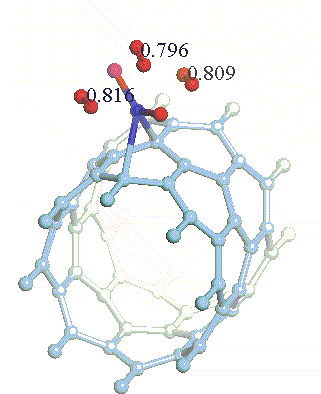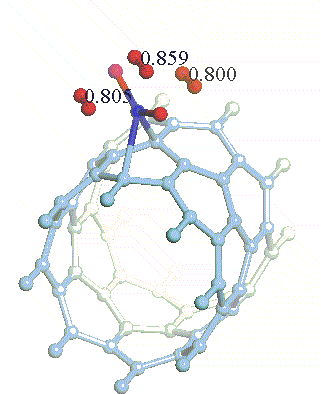 There have been a great number of reports on the search of new routes to engineer nanomaterials so that (1) they dissociate H2 molecules into H atoms and (2) reversibly adsorb hydrogen molecules at ambient conditions. Much effort has been focused on the engineering of carbon-based materials such as nanotubes and transition metal hydrides without success. It is found that while hydrogen-carbon interaction is too weak, the metal-hdyrogen interaction is too strong for room temperature reversible storage. Here we show a novel way to overcome this difficulty by forming artificial metal-carbides on nanotubes/C60 molecules! From accurate first-principles calculations, we show that a single Ti-atom coated on a SWNT/C60 can strongly adsorb up to four hydrogen molecules. The hydrogen-metal bonding is an unusual combination of chemi- and physi-sorption, an essential ingredient needed for reversible hydrogen storage medium near room temperature. Remarkably, this adsorption occurs with no energy barrier.
There have been a great number of reports on the search of new routes to engineer nanomaterials so that (1) they dissociate H2 molecules into H atoms and (2) reversibly adsorb hydrogen molecules at ambient conditions. Much effort has been focused on the engineering of carbon-based materials such as nanotubes and transition metal hydrides without success. It is found that while hydrogen-carbon interaction is too weak, the metal-hdyrogen interaction is too strong for room temperature reversible storage. Here we show a novel way to overcome this difficulty by forming artificial metal-carbides on nanotubes/C60 molecules! From accurate first-principles calculations, we show that a single Ti-atom coated on a SWNT/C60 can strongly adsorb up to four hydrogen molecules. The hydrogen-metal bonding is an unusual combination of chemi- and physi-sorption, an essential ingredient needed for reversible hydrogen storage medium near room temperature. Remarkably, this adsorption occurs with no energy barrier.
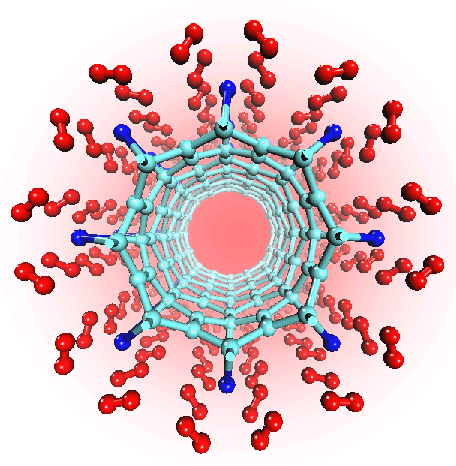
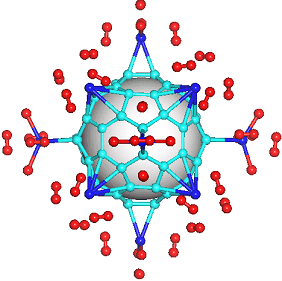 At large Ti coverage we show that a (8,0) SWNT and C60 can store hydrogen molecules up to 8-wt%, exceeding the minimum requirement of 6-wt% for practical applications. Finally, we present high temperature quantum molecular dynamics simulations showing that these systems are stable and indeed exhibit associative desorption of H2 upon heating, another requirement for reversible storage.These results are quite remarkable, unanticipated, and to the best of our knowledge, the first-demonstration of hydrogen-metal complex formation containing FOUR H2 molecules.
SEE FULL STORY..
At large Ti coverage we show that a (8,0) SWNT and C60 can store hydrogen molecules up to 8-wt%, exceeding the minimum requirement of 6-wt% for practical applications. Finally, we present high temperature quantum molecular dynamics simulations showing that these systems are stable and indeed exhibit associative desorption of H2 upon heating, another requirement for reversible storage.These results are quite remarkable, unanticipated, and to the best of our knowledge, the first-demonstration of hydrogen-metal complex formation containing FOUR H2 molecules.
SEE FULL STORY..
 In this study, we have used neutron scattering techniques to visualize the available adsorption sites in MOF materials directly. Fourier difference analysis of the neutron diffraction data clearly show that the molecules are packed in a fashion similar to the way apples or oranges fill a bowl. Surprisingly, we found that the MOF host lattice has the right-space available to hold many hydrogen molecules, up to ~10 wt. % at low temperatures. The metal-oxide cluster is responsible for most of the adsorption while the organic linker plays only a secondary role. These results suggest that by using different organic linkers, which make the hydrogen desorption difficult by narrowing the channels connecting the network of nano-pores in MOF, one may be able to optimize these materials for practical hydrogen storage at ambient conditions.
Equally important, we show that at high-concentration loadings hydrogen molecules form unique 3D networks of hydrogen nano-clusters with intermolecular distances as small as 3.0 Å, which in pure solid hydrogen can only be reached at high pressure. These findings suggest that MOF materials can also be used as templates to create artificial, interlinked hydrogen nano-cages. Such materials could exhibit very unexpected electronic properties due to these small intermolecular distances, such as metallic or superconducting behavior.
SEE FULL STORY..
In this study, we have used neutron scattering techniques to visualize the available adsorption sites in MOF materials directly. Fourier difference analysis of the neutron diffraction data clearly show that the molecules are packed in a fashion similar to the way apples or oranges fill a bowl. Surprisingly, we found that the MOF host lattice has the right-space available to hold many hydrogen molecules, up to ~10 wt. % at low temperatures. The metal-oxide cluster is responsible for most of the adsorption while the organic linker plays only a secondary role. These results suggest that by using different organic linkers, which make the hydrogen desorption difficult by narrowing the channels connecting the network of nano-pores in MOF, one may be able to optimize these materials for practical hydrogen storage at ambient conditions.
Equally important, we show that at high-concentration loadings hydrogen molecules form unique 3D networks of hydrogen nano-clusters with intermolecular distances as small as 3.0 Å, which in pure solid hydrogen can only be reached at high pressure. These findings suggest that MOF materials can also be used as templates to create artificial, interlinked hydrogen nano-cages. Such materials could exhibit very unexpected electronic properties due to these small intermolecular distances, such as metallic or superconducting behavior.
SEE FULL STORY..





 NRC Postdoc Needed!.
NRC Postdoc Needed!.