|
|
Agency for Toxic Substances and Disease Registry
Polychlorinated Biphenyls (PCB) Toxicity
Exposure Pathways
PCBs are the family of chemicals formed by attaching one or more chlorine atoms to a pair of connected
benzene rings (Figure 1). Depending on the number and position of chlorine atoms attached to the biphenyl ring structure, 209 different PCB congeners can be formed. The chemical and toxicologic properties of PCBs vary from one congener to the next.
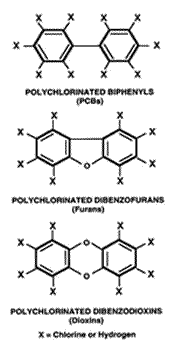
Figure 1.
Because of their insulating and nonflammable properties, PCBs were marketed for nearly 40 years (ATSDR 2000a) as heat exchange and dielectric fluids in transformers and capacitors; hydraulic and lubricating fluids; diffusion pump oils; plasticizers; extenders for pesticides; and as ingredients in caulking compounds, paints, adhesives, and flame retardants. PCBs have also been used in inks and carbonless carbon paper. Commercial PCB products were always mixtures of different PCB congeners and were usually contaminated with small amounts of polychlorinated dibenzofurans (furans) or polychlorinated dibenzodioxins (dioxins). Contamination is a concern because the toxicity of contaminants is generally much greater than that of PCBs. Trade names for commercial PCB mixtures included Aroclor, Askarel, Eucarel, Pyranol, Dykanol, Clorphen, Asbestol, Diaclor, Nepolin, and EEC-18.
No known natural sources of PCBs exist. Production of these chemicals was banned in 1977, when their ability to accumulate in the environment and to cause harmful effects became apparent (ATSDR 2000a). Today, the major source of ambient PCB exposure seems to be environmental cycling of PCBs previously released into the environment. Of the 1.25 billion pounds of PCBs produced in this country between 1929 and 1977, about 450 million pounds have found their way into the environment. PCBs can be released into the general environment from poorly maintained toxic waste sites; by illegal or improper dumping of PCB wastes, such as transformer fluids; through leaks or fugitive emissions from electrical transformers containing PCBs; and by disposal of PCB-containing consumer products in municipal landfills. PCBs have been found in at least 432 of the 1,467 hazardous waste sites on the U.S. Environmental Protection Agency (EPA) National Priorities List (NPL) (ATSDR 2000a), and low levels of PCBs can be found throughout the world.
- PCBs persist in the environment, concentrating upward in the food chain.
Once released into the environment, PCBs adsorb strongly to soil and sediment. As a result, these compounds tend to persist in the environment, with half-lives for most congeners ranging from months to years. Leaching of PCBs from soil is slow, particularly for the more highly chlorinated congeners, and translocation to plants via soil is insignificant. Cycling of PCBs through the environment involves volatilization from land and water surfaces into the atmosphere, with subsequent removal from the atmosphere by wet or dry deposition, then revolatilization (ATSDR 2000a). Inhalation of these volatilized compounds is one possible route of exposure in the general population, but it is not the primary route of exposure.
- The primary nonoccupational source of PCB exposure is food, especially fish from contaminated waters.
The primary route of exposure to PCBs in the general population appears to involve the consumption of contaminated foods, particularly meat, fish, and poultry (ATSDR 2000a). In aquatic environments, the high lipophilicity of PCBs causes these compounds to partition out of the water and become preferentially adsorbed to sediments. Although sediment adsorption is useful in preventing the contamination of drinking water supplies, the partitioning of PCBs to sediments plays a role in the tendency of these compounds to become concentrated in aquatic organisms. Bottom-feeding fish ingest and accumulate PCBs from sediment. The resistance of these compounds to biodegradation causes PCBs to become more concentrated as they move upward through the food chain. As a result of this bioconcentration, PCB levels in aquatic organisms can be up to 1 million times higher than their concentration in the aquatic environment. In the National Study of Chemical Residues in Fish conducted between 1986 and 1989 (EPA 1992a, 1992b), the mean concentration of PCBsin bottom-feeding and game fish was 1.9 parts per million (ppm). However, PCB levels as high as 20 ppm have been detected in game fish taken from waters near hazardous waste sites (ATSDR 2000a).
Although occupational exposure no longer occurs as a result of the manufacture of PCB-containing products, it might still occur during the maintenance or repair of equipment that contains PCBs or as a result of accidents involving such equipment (ATSDR 2000a). Today, PCBs are found mainly in transformers and capacitors manufactured before 1977. Such transformers and capacitors might be found in old industrial equipment (e.g., welding equipment), medical equipment (e.g., X-ray machines), and household appliances (e.g., refrigerators and televisions). The ballasts of older fluorescent light fixtures might also contain PCBs. During normal operation of these lights, the PCBs are entirely enclosed; when the capacitor wears out, however, it can burn or break and leak PCBs.
Occupational exposure to PCBs occurs mainly via the inhalation and dermal routes. Commercial PCB mixtures are colorless to dark brown oils, viscous liquids, or sticky resinous semisolids. Although they evaporate slowly at room temperature, the volatility of PCBs increases dramatically with even a small increase in temperature. Overheated equipment that contains PCBs can vaporize significant quantities of these compounds, creating an inhalation hazard that can be magnified by poor ventilation. Because of their highly lipophilic nature, PCBs can also be absorbed through the skin following contact with contaminated equipment, water, or soil.
| In response to your persistent, detailed questioning about his work, hobbies and recreational activities, and possible contact with hepatotoxins, the patient reveals that while in the basement workshop he frequently wipes up a dark, oily discharge near a large electrical transformer that he collected in the work area. The discharge has also resulted in a gummy residue on tools and other surfaces. He mentions he sometimes feels dizzy and nauseated after working in the basement all day. |
| 1. |
Is there an association between the clinical findings and this additional information? |
Previous Section
Next Section
|




