|
Accelerating Progress in Cancer Prevention
One way to eliminate the suffering and death due to cancer is to develop ways to prevent the disease. Toward
this end, NCI and its international collaborators are seeking to identify medical, behavioral, and environmental
approaches to cancer prevention that can be translated effectively to public health settings. Basic biomedical
research relevant to the prevention of cancer is one avenue of investigation. Exploring strategies to modify
behaviors that can increase a person's risk of cancer - such as poor diet, physical inactivity, excessive sun
exposure, and tobacco use - is a second. How to mitigate the influence of environmental risk factors, including
occupational exposures and infectious agents, is yet another.
The following summaries highlight some of NCI's international activities in the area of cancer prevention.
In 1996, the Director-General of the World Health Organization (WHO) was called upon
to initiate development of a Framework Convention on Tobacco Control (FCTC). The
resulting international convention - the first global health treaty negotiated under the
auspices of WHO - was adopted unanimously by the World Health Assembly in 2003
and entered into force in 2005. Thus far, 126 nations have ratified, accepted, approved,
formally confirmed, and acceded to the treaty.
The WHO's Tobacco Free Initiative (TFI) was established in this context, with the objective
of reducing the global burden of disease and death caused by tobacco. Under the TFI,
the WHO created a scientific advisory committee, called the Scientific Advisory Committee
on Tobacco Product Regulation (SACTob), to provide scientifically sound recommendations
regarding the most effective and evidence-based means to achieve a coordinated regulatory
framework for tobacco products. In 2003, SACTob became a formal study group,
called the Study Group for Tobacco Regulation (TobReg). TobReg provides a formal
mechanism for reporting to WHO's Executive Board in order to draw the attention of
member nations to WHO's efforts in tobacco regulation. NCI scientists have worked with
SACTob/TobReg since 2002 on the development of numerous recommendations aimed at
improving public health and scientific research related to the effects of tobacco use.
 In addition, NCI participated in the TFI effort in 2004 to create
an International Network for Tobacco Testing and Research for
Regulation (INTTARR) to address research issues related to the
development of a global capacity for tobacco product testing and
research. INTTARR, which has since been renamed the Tobacco
Laboratory Network (TobLabNet), is a global network of government,
university, and independent laboratories across the world to
advance research on tobacco product testing. Testing and measuring
tobacco products at the national or regional level are essential
to monitoring compliance by tobacco manufacturers of their
obligations under the FCTC to test and disclose the contents and
emissions of their products. Regulators must also have the capacity
to test and measure tobacco products in order to propose tobacco product content and
emissions regulations in the future. Furthermore, a capacity for testing and research is
one of the factors needed to ensure manufacturers package and label their products in a
manner that does not mislead consumers about the health risks of tobacco.
In addition, NCI participated in the TFI effort in 2004 to create
an International Network for Tobacco Testing and Research for
Regulation (INTTARR) to address research issues related to the
development of a global capacity for tobacco product testing and
research. INTTARR, which has since been renamed the Tobacco
Laboratory Network (TobLabNet), is a global network of government,
university, and independent laboratories across the world to
advance research on tobacco product testing. Testing and measuring
tobacco products at the national or regional level are essential
to monitoring compliance by tobacco manufacturers of their
obligations under the FCTC to test and disclose the contents and
emissions of their products. Regulators must also have the capacity
to test and measure tobacco products in order to propose tobacco product content and
emissions regulations in the future. Furthermore, a capacity for testing and research is
one of the factors needed to ensure manufacturers package and label their products in a
manner that does not mislead consumers about the health risks of tobacco.
Finally, NCI contributes to a multi-agency collaboration, which includes five other institutes
of the National Institutes of Health (NIH), the NIH's Fogarty International Center,
and the TFI, that funds research on tobacco use and related illness in developing countries.
The tobacco industry has exerted substantial influence on tobacco policies throughout the
world, and it is important to understand this influence as we strive to reduce tobaccorelated
disease.
NCI-supported investigators at the University of London's School of Hygiene and
Tropical Medicine are analyzing efforts made by the tobacco industry to influence tobacco
control policies in selected countries, regions, and around the world. These investigators
are using industry documents housed at the Guildford Depository in the United
Kingdom to compile country profiles of tobacco industry activities in 14 countries.
The Guilford Depository was established as a consequence of litigation brought against
several tobacco companies by the State of Minnesota and Minnesota Blue Cross Blue
Shield. The parties settled in 1998, with the agreement of the Minnesota Consent
Judgment, in which the British American Tobacco Company agreed to provide public
access to the internal documents it produced during the discovery process. The documents
were to be made available at the Guildford Depository for a period of 10 years, which
expires in February 2009.
Analysis of the country profiles is offering valuable insights into how the tobacco industry
may have influenced tobacco-related, public policy-making, and scientific research efforts
in the countries being studied. This information is also enabling investigators to examine
the connections between globalization, the tobacco industry, and policy influence, and to
develop recommendations about how to create more effective tobacco-control strategies
and policies to prevent and reduce tobacco use.
In 1999, NCI, the National Institute on Drug Abuse, and the Robert Wood Johnson
Foundation, created the TTURCs to facilitate a transdisciplinary approach to the full
spectrum of basic and applied research on tobacco use. The goal of research projects conducted
through the TTURCs is to help reduce the burden of tobacco-related diseases. In
2004, the National Institute of Alcohol Abuse and Alcoholism joined as a funding partner,
and two TTURCs are currently investigating tobacco use in international populations.
Scientists affiliated with the University of Southern California's Pacific Rim TTURC
have previously studied multiethnic and multicultural populations within the United
States and in China to explore cultural, social, psychological, and environmental factors
that influence tobacco use by adolescents. This research indicated that cultural context
and individual disposition can moderate the effectiveness of prevention programs.
Investigators at the center are now conducting three projects that build on the earlier
findings: 1) a study of substance use by 600 adolescent twin pairs in Southern California
and another 600 adolescent twin pairs in Qingdao, China; researchers will examine
social-environmental and heritable risk and protective factors for tobacco and alcohol
use among the adolescents; 2) a study of why school- and community-based smoking prevention
programs work in some situations and not in others; researchers will study the
effects that a student's cultural and environmental context and dispositional characteristics
(particularly, hostility and depression) have on substance use, and how these factors
influence the effectiveness of prevention and cessation programs; and 3) a study to investigate
the hypothesis that genetic factors responsible for a person's dispositional attributes
(e.g., hostility and depression) may substantially influence both the individual's tobacco
use and the effectiveness of tobacco-control intervention and prevention programs.
The Roswell Park Cancer Institute's TTURC will expand the ongoing International
Tobacco Control Policy Evaluation Survey (ITCPES), which is a longitudinal study of
smokers in the United States, Canada, the United Kingdom, and Australia. The expanded
study will include smokers in Ireland, Thailand, and Malaysia. This expansion will allow
researchers to assess whether tobacco control and prevention policies that are effective
in developed countries are equally effective in developing nations. The researchers will
also conduct follow-up surveys of the 8,300 smokers from the four countries that initially
participated in the ITCPES to evaluate whether comprehensive tobacco control policies
being implemented in these countries are effective in reducing tobacco use. The results of
this TTURC project can provide insights into how and why specific policies influence
tobacco-related behaviors.
|
The prevention of cervical cancer is emerging as a major public health advance; one that has implications for
women throughout the world. Worldwide, cervical cancer causes more than 200,000 deaths each year, and approximately
80 percent of the women who die from this disease live in developing countries. Over the past 30 years,
cervical cancer deaths have declined markedly in the United States - a decline that is due, in large measure, to
effective screening programs. These programs, unfortunately, are quite rare in developing countries. Yet, we now
know that infection with human papillomavirus, or HPV, is the principal cause of cervical cancer in this country
and abroad. This knowledge has made vaccine therapy a viable option for cervical cancer prevention.
Two pharmaceutical companies - Merck and Co, Inc. and GlaxoSmithKline Biologicals (GSK) - have recently
produced vaccines against HPV. Both vaccines are based on technology developed by NCI scientists, whose work
laid the foundation for the production of HPV "virus-like particle," or VLP, vaccines. VLPs contain the L1 outer
coat protein of HPV, yet are noninfectious. They are produced in insect cells or yeast cells by recombinant
DNA technology. The cells make large amounts of the L1 protein, which then self assembles into particles that
look like HPV but do not contain the virus' genetic material.
In a randomized clinical trial, the GSK vaccine - called CervarixTM - provided nearly complete protection against
infections caused by HPV-16 and HPV-18, two strains of HPV that are responsible for 70 percent of all cervical
cancers. The vaccine, which was also highly effective against persistent infections caused by the two strains,
contains a mixture of HPV-16 and HPV-18 VLPs.
Approximately 1,100 women from Canada, Brazil, and the United States participated in the HPV vaccine trial.
These women were randomly assigned to receive doses of the HPV-16/18 vaccine or a placebo initially and then
at 1 month and 6 months after their initial injection. The researchers found that the GSK vaccine was 91.6 percent
effective in protecting against incident infections with HPV-16 or HPV-18 in women treated according to the trial
protocol. The vaccine was 100 percent effective in preventing persistent viral infections during the study period,
and it was 93.5 percent effective in preventing cervical cell abnormalities associated with HVP-16 or HPV-18
infection. Additional follow-up of participants in this trial has demonstrated that effective protection against
infection remains high for up to 4 years. Longer-term efficacy of the vaccine is still not known.
In another clinical trial, the vaccine developed by Merck was found to be nearly completely effective in preventing
incident and persistent infections with HPV-16 and HPV-18 and against cervical cell abnormalities associated with
HPV-16 or HPV-18. This vaccine - called GardasilTM - also protected against two other HPV strains, HPV-6 and
HPV-11, which cause 90 percent of genital warts. More than 12,000 women from 13 countries participated in the
trial. GardasilTM was administered in three doses over 6 months and provided 100 percent protection against
HPV infection for the 18-month duration of the study. The long-term efficacy of the vaccine is still not known, and
follow-up studies will be required to answer this question.
NCI is now conducting a Phase III clinical trial in Costa Rica - where cervical cancer rates are high - to further
test the HPV 16/18 vaccine developed by GSK. Over 7,400 women have been enrolled in the trial. They will be
followed for at least 4 years to allow investigators to gather information about the vaccine's long-term safety and
efficacy. NCI investigators also plan to evaluate other potential effects of the vaccine, including: 1) its effectiveness
against additional HPV strains; 2) its ability to speed the healing of established cervical infections; and 3) evaluate
the immune mechanisms of long-term protection. The NCI trial in Costa Rica will also provide important information
that will be useful to evaluate the cost-effectiveness of HPV vaccination and combined vaccination and screening
prevention efforts.
Harper D, Franco E. Wheeler C, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho N, Roteli-Martins C, Teixeira J, Blatter M, Korn A, Quint W, Dubin G.
Efficacy of a bivalent L1virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled
trial. Lancet, November 13, 2004; 364(9447):1757-1765.
Harper D, Franco E, Wheeler C, Moscicki A, Romanowski B, Roteli-Martins C, Jenkins D, Schuind A, Clemens S, and Dubin G on behalf of the HPV Vaccine Study
group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised
control trial. Lancet, April 15, 2006;367(9518):1213-1290.
Finn S, et al. Prophylactic quadrivalent human papillomavirus (HPV) (types 6, 11, 16, 18) L1 virus-like particle (VLP) vaccine (GardasilTM) reduces cervical intraepithelial
neoplasia (CIN) 2/3 risk. Oral abstract LB-8a. Infectious Diseases Society of America meeting. San Francisco, CA. October 7, 2005.
Villa L, Costa R, Petta C, Andrade R, Ault K, Giuliano A, Wheeler C, Koutsky L, Malm C, Lehtinen M, Skjeldestad F, Olsson S, Steinwall M, Brown D, Kurman R,
Ronnett B, Stoler M, Ferenczy A, Harper D, Tamms G, Yu J, Lupinacci L, Railkar R, Taddeo F, Jansen K, Esser M, Sings H, Saah A, Barr E. Prophylactic quadrivalent
human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre Phase II
efficacy trial. Lancet Oncology, May 1, 2005;6(5):271-278.
|
People can be exposed to two types of phytoestrogens through their diet, isoflavones and
lignans. Isoflavones are found in soy and soy products, and lignans are found in whole
grains, fruits, and vegetables. Some evidence suggests that these agents may protect
against hormone-dependent cancers; however, human studies to date have yielded only
inconclusive results. Large-scale, prospective studies are needed to fully evaluate whether
dietary phytoestrogens are effective in preventing cancer. Such studies, however, are
expensive and difficult to undertake.
NCI is supporting a pilot study, using a subgroup of participants from the European
Prospective Investigation into Cancer and Nutrition (EPIC) project, to determine whether
a large-scale prospective study of phytoestrogens is justified. The EPIC project - the
largest study of diet and health ever undertaken - has registered more than 500,000 men
and women aged 35 to 69 years from 10 different European countries. The pilot study
will examine variations in plasma phytoestrogen levels in 1,600 randomly selected EPIC
participants from 17 different regions across Europe. In particular, plasma levels of the
isoflavones genistein, daidzein, glycetin, and equol and the lignans enterolactone and
enterodiol will be measured. The influence of age, gender, geographic region, and habitual
diet on the plasma levels of these phytoestrogens will also be determined.
Factors associated with reproduction likely play an important role in the development of
ovarian cancer. During pregnancy, the placenta produces large amounts of the female
sex hormones estrogen and progesterone. A number of factors, including gestational age
of the developing fetus, affect the levels of these hormones during pregnancy. Although
research has shown that increasing parity (number of births) reduces a woman's risk of
ovarian cancer, no study to date has examined the associations between indictors of hormonal
exposures during pregnancy and subsequent ovarian cancer risk.
NCI is collaborating with the Karolinska Institute to conduct a large cohort study aimed
at exploring these associations. Investigators are using information gathered on more than
1.2 million women who delivered their first infant between 1973 and 2000 and who
were included in the nationwide Swedish Medical Birth Register. Researchers will: 1)
study ovarian cancer risk using markers of hormone exposures during pregnancy, including
birth weight, gestational age, single or multiple birth, pregnancy-induced hypertensive
diseases, and placental weight; 2) examine whether the protective effects of increasing
parity and a high age at first birth are influenced by markers of hormone exposures
during pregnancy; and 3) assess the importance of other factors on ovarian cancer risk.
The investigators plan to gather information about maternal characteristics, pregnancy
complications, placental weight, and birth characteristics for all births associated
with the women in the cohort. In addition, information about gynecologic surgeries,
vital status, and ovarian cancer incidence for these women will be collected from
population-based registries.
Ovarian cancer accounts for approximately 4 percent of all cancers among women
worldwide and has the highest mortality of all cancers of the female reproductive system.
Use of the Swedish research registries in this study allows a large, cost-effective study
to be conducted where information has been prospectively collected about markers of
hormone exposure during pregnancy.

|
| Unhealthy, High-fat,
High-carbohydrate |
Living a Western lifestyle, which is characterized by a low level of
physical activity and an energy-dense diet rich in easily digestible
(refined) carbohydrates and fats, is associated with an increased risk
of colon cancer. Etiologic models to explain this association have
focused mostly on the effects of diet in exposing the colonic mucosa
to mutagenic or tumor-promoting compounds. The results of this
study should allow formulation of more precise nutritional guidelines
for the effective prevention of colon cancer.
Several NCI-sponsored clinical trials are currently testing the effectiveness of celecoxib
(Celebrex®) in preventing a variety of cancers. Celecoxib, used to treat osteoarthritis and
adult rheumatoid arthritis, reduces inflammation by blocking the activity of the cyclooxygenase-
2, or COX-2, enzyme. The COX-2 enzyme is activated only during inflammation,
and evidence suggests that elevated levels of COX-2 may contribute to the development
of a variety of cancers, including esophageal, stomach, colon, pancreas, liver, breast, lung,
bladder, cervical, and head and neck cancers.
NCI and Pfizer, Inc., jointly sponsored the Adenoma Prevention with Celecoxib (APC) Trial
to investigate whether celecoxib could reduce the occurrence of new colorectal adenomas
(precancerous polyps) in people who already had such a polyp removed. More than 90 centers
- located in the United States, the United Kingdom, Australia, and Canada - and more
than 2,000 men and women age 30 years or older participated in this trial. From November
1999 to March 2002, study participants were randomly assigned to take either 200 mg of
celecoxib twice a day, 400 mg of celecoxib twice a day, or a placebo twice a day for 3 years.
Initial results of the trial were reported in April 2006 and showed that those taking celecoxib
had 33 to 45 percent fewer new adenomas and 57 to 66 percent fewer high-risk adenomas
than those taking the placebo. When adenomas recurred in the participants taking celecoxib,
the growths were fewer and smaller than those in the participants who took the placebo.
In December 2004, use of celecoxib in the APC Trial was suspended because an analysis
by an independent data safety and monitoring board showed that participants taking
the drug had a 2.5-times greater risk of fatal and major non-fatal cardiovascular events
(cardiovascular death, heart attack, stroke, or heart failure) than those on placebo. In
February 2005, the APC investigators published a full analysis of the cardiovascular
events, reporting that celecoxib use for an average of almost three years was associated
with a dose-related increased risk of serious events.
In view of the increased risk of serious cardiovascular events found in the APC trial,
NCI notified all principal investigators of its sponsored trials involving COX-2 inhibitors
and asked them to inform their institutional review boards, data safety and monitoring
boards, and trial participants about this new information. NCI also required that the
informed consent forms for the trials be revised to reflect the new information. Trial
participants were asked to sign new consent forms with updated information about the
risks and benefits of the trials.
A Phase II trial comparing the effectiveness of celecoxib, taken alone or in combination with
eflornithine, in preventing colorectal cancer in patients with familial adenomatous polyposis
(FAP) is currently underway in the United States and the United Kingdom. FAP is an inherited
disorder that is characterized by the development of numerous polyps in the colon and rectum.
People diagnosed with FAP are at increased risk of colon cancer. Although most FAP patients
undergo colectomy (surgical removal of all or part of the colon), researchers are interested in
developing drugs that may offer an additional measure of protection to individuals with this
condition. In the trial, 120 patients between the ages of 18 and 65 who have been diagnosed
with FAP will be randomly assigned to receive celecoxib alone or celecoxib plus eflornithine.
Eflornithine, also known as alpha-difluoromethylornithine, is an inhibitor of the enzyme
ornithine decarboxylase (ODC). Inhibitors of ODC have been shown to suppress tumor
formation in experimental models of bladder, breast, colon, and skin carcinogenesis.
Esophageal squamous cell carcinoma (ESCC) is the third most common cancer of the
digestive tract and the seventh leading cause of cancer-related deaths worldwide. The
incidence of this cancer varies greatly according to geographic location. It is more
common in Northern China, Iran, and the southern regions of the former Soviet
Republic and is less common in Japan, Europe, and Canada. Patients with ESCC are
often diagnosed when the cancer is advanced. However, clinicians have noted that
premalignant lesions can precede the onset of ESCC, and these lesions may represent
a potential target for prevention efforts.
NCI-funded scientists recently completed a randomized, placebo-controlled chemoprevention
trial among people in Lixian, China, who had mild or moderate premalignant disease
and were, therefore, considered to be at high-risk for ESCC. The study participants were
randomly assigned to receive selenomethionine alone, celecoxib alone, a combination of
selenomethionine and celecoxib, or a placebo over a period of 10 months. A total of 360
individuals were randomized to the four groups; 238 individuals were included in the
final analysis. The results of the trial were reported in 2005.
Overall, there was a trend toward increased regression and decreased progression of
premalignant lesions in selenomethionine-treated subjects in comparison with those not
treated with selenomethionine, but the results were not statistically significant. In
unplanned analyses, treatment with selenomethionine favorably affected a change in
dysplasia grade among the 115 subjects who had mild premalignant disease at baseline
but not among the 123 subjects who had moderate premalignant disease at baseline.
Treatment with celecoxib had no effect on disease regression or progression. This is the
first report of a potential chemoprevention agent for ESCC.
|
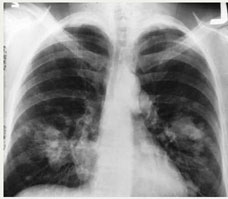 |
| This is an x-ray image
of a chest. Both sides
of the lungs are visible
with a growth on the
left side of the lung,
which could possibly
be lung cancer. |
As the number of former smokers increases, so does the need to find
effective interventions for preventing lung cancer in this high-risk population.
Inhalers have long been considered a safe and effective way to
deliver medications for treating chronic asthma. Now, scientists are testing
whether inhalers can safely and effectively deliver agents to prevent
lung cancer. Scientists are now evaluating whether inhaled budesonide,
a steroid commonly used to treat asthma, can prevent the development
of lung cancer.
In preclinical studies, NCI investigators found evidence that inhaled
budesonide may impede lung tumor growth if it is given in the early
stages of tumor development. The studies showed that glucocorticoids,
which include budesonide, were nearly 90 percent effective in preventing
lung adenomas in mice. These results served as the foundation for a Phase II clinical trial, in
which a group of Canadian smokers took inhaled budesonide for 6 months. In this trial, which
was reported in 2004, the drug had no effect on the growth of bronchial lesions or the prevention
of new lesions. However, spiral computed tomography (CT) scans performed on the trial participants
revealed that budesonide may have affected small nodules - some of which may have
been precancerous - in the peripheral lungs.
On the basis of this observation, a new clinical trial testing inhaled budesonide recently began
in Italy. This Phase II trial, supported by NCI and led by the European Institute of Oncology, will
enroll individuals who are already receiving annual spiral CTs as part of a larger lung cancer
screening trial. The researchers will focus on individuals who, after the second annual CT, have
persistent lung nodules that may be precursors to lung adenocarcinomas. These individuals will
be treated for 1 year with either budesonide or a placebo. If the budesonide is found to be an
effective chemopreventive agent for the trial participants - that is, it causes lung nodules to
regress - a larger trial will follow. The investigators will also assess whether this drug is safe
for those who are at higher risk of developing lung cancer.
Inhalers have the advantage of delivering medication directly to the lungs, limiting the risk of
potential side effects in other parts of the body. Because it is a targeted delivery system, it can
reduce overall toxicity. However, an agent to prevent lung cancer will probably need to be taken
over an extended period of time, therefore the long-term toxicity of the agent is a critical concern.
|
In 2003, NCI, the Centers for Disease Control and Prevention, and the Substance Abuse
and Mental Health Services Administration, jointly developed and launched a Web portal
called Cancer Control PLANET (Plan, Link, Act, Network with Evidence-based Tools).
This Web portal serves as a doorway to new evidence-based tools - developed through a
public-private effort involving these federal agencies and the American Cancer Society -
that can help communities better understand and address their cancer burden. Cancer
Control PLANET is organized around five steps that U.S. communities can take to develop
a comprehensive cancer control plan.
A prototype of an international version of the Cancer Control PLANET Web portal will
be demonstrated at the 2006 International Cancer Control Conference, in conjunction
with the UICC World Cancer Congress in Washington, D.C. Full implementation of the
International Cancer Control PLANET Web portal is anticipated in 2007, with ongoing
NCI involvement in updating international data for the portal in future years.
Back to Top
< Previous Section | Next Section > |