![]()
This article originally appeared in the October 1996 FDA Consumer.
The version below is from a reprint of the original article and contains revisions made in September 1997.
The Food and Drug Administration has recently approved three new automated systems that show promise of substantially improving the accuracy of Pap tests.
A Silent Cancer
Unlike many cancers that cause pain, noticeable lumps, or other early symptoms, cervical cancer has no telltale symptoms until it is so advanced that it is usually unresponsive to treatment. Symptoms may even be absent at that point, although they often include abnormal vaginal bleeding, such as following intercourse or douching, between menstrual periods, or after menopause. Only in its late stages does cervical cancer cause pain in the lower abdominal or back regions.
But because the cervix, or neck of the uterus, can be easily accessed through the vagina, doctors can test for cervical cancer as well as for precancerous changes in the cervix. Most cervical cancers grow slowly over several years and often are preceded by abnormal cells. Cervical cancer can often be prevented by the removal of these abnormal cells.(See accompanying article.)
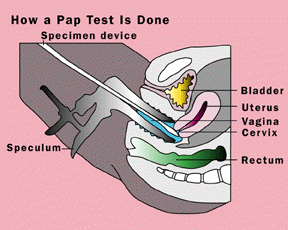 To detect abnormal or cancerous cervical cells, George Papanicolaou, M.D., Ph.D., of Cornell University developed in the 1940s what is known today as the Pap test. In this test, a sample of cells is taken from in and around the cervix with a wooden scraper, cotton swab, or small cervical brush. The specimen is smeared on a glass slide, preserved with alcohol, and then sent to a laboratory. There cytotechnologists, specially trained in identifying abnormal cells, scrutinize the cervical cells under the microscope for any abnormal features associated with cancerous or precancerous cervical cells. These features include dark or irregularly shaped cell nuclei, or small or deformed cells.
To detect abnormal or cancerous cervical cells, George Papanicolaou, M.D., Ph.D., of Cornell University developed in the 1940s what is known today as the Pap test. In this test, a sample of cells is taken from in and around the cervix with a wooden scraper, cotton swab, or small cervical brush. The specimen is smeared on a glass slide, preserved with alcohol, and then sent to a laboratory. There cytotechnologists, specially trained in identifying abnormal cells, scrutinize the cervical cells under the microscope for any abnormal features associated with cancerous or precancerous cervical cells. These features include dark or irregularly shaped cell nuclei, or small or deformed cells.
The Pap test became a routine part of gynecological exams. As a result, there was a 70 percent drop in the number of women dying from cervical cancer between 1950 and 1970, according to the National Cancer Institute. But the problem of errors remained. Such errors are understandable when considering the magnitude of the task set before the cytotechnologist examining Pap slides. These standard-sized laboratory slides are lined with between 50,000 to 300,000 cervical cells. Lurking in these cells may be as few as a dozen abnormal cells. Finding such telltale cells is akin to finding a needle in a haystack, especially at the end of the day when cytotechnologists are likely to have examined nearly 100 Pap slides. In addition, abnormalities in cell shape may be slight and difficult for even the trained eye to detect, or may be masked by infection.
Improving Detection
A new slide preparation method may improve the accuracy of the initial screening.
The ThinPrep Processor Model 2000 is an automated slide preparation system for Pap smears that may make it easier to screen for atypical cells. Safety and effectiveness data submitted by the manufacturer to FDA demonstrated that slides prepared with the ThinPrep system are significantly more effective in a variety of patient populations for detecting low-grade squamous intraepithelial lesions (SILs) and some of the more severe lesions. In addition, the ThinPrep Processor is as safe and effective as the conventional method of preparing slides by hand for detecting all categories of atypical and diseased cervical cells.
In this system, improved quality comes from an automated process that concentrates the cellular material and filters out a lot of blood and other unneeded material.
The other new systems are computerized rescreening methods. In both systems, computers scan the slides for abnormal-looking cells.
One system, called PAPNET, uses neural net computer technology, which its manufacturer claims was originally created to detect flying missiles in what is known as the "Star Wars" defense strategy. PAPNET detects abnormal cervical cells with a computer system that essentially has learned by example. This system was created by feeding a series of digitized images of Pap slides to a computer. From these examples, the computer developed the guidelines for predicting abnormal cells.
PAPNET scans each Pap slide cytotechnologists have classified as normal and chooses the 128 cells or cell clusters that are most likely to be abnormal. Enlarged color images of these cells are then returned to the cytotechnologist for review.
Studies have used PAPNET rescreening to reexamine previous negative Pap smears taken from women with high-grade cervical cell abnormalities or cervical cancers. These studies found that in about one-third of these women, PAPNET testing detected abnormalities missed by manual screening on previous Pap smears.
The other Pap test rescreening system is called AutoPap 300 QC. This computerized system uses image interproduction and pattern recognition techniques to classify cells as abnormal. Hundreds of features--such as size, shape, density, and texture--are considered for each cell. Sophisticated statistical screens use this visual information to predict which cervical cells are abnormal. Following routine screening by a cytotechnologist, all "normal" slides are rescreened by AutoPap 300 QC, which selects 10 to 20 percent of slides with the highest probability of having abnormal cells. These are then rescreened manually by the cytotechnologist.
In one study, cytotechnologists randomly rescreening 10 percent of more than 4,000 Pap slides they originally classified as normal detected only about 1 of every 10 false negatives present. Cytotechnologists using AutoPap 300 QC to select the 10 percent of slides the system deemed as being most abnormal detected up to half of all the missed abnormals.
All three products are available for use by laboratories, but some labs may not yet be fully familiar with these new systems.
"Laboratories are starting to evaluate these devices and determine if and how they will use them," said Louise Magruder, of FDA's division of clinical laboratory devices.
Although use of ThinPrep, PAPNET and AutoPap 300 QC will considerably decrease the likelihood of missing a diagnosis of cervical cancer, none of these systems is perfect. Even if the rescreening systems could detect every abnormal cell on a Pap slide, some women with cervical cancer would still be told their Pap tests were normal because there were too few cells on the slide or the cell samples were not taken from both the inside and surface of the cervix. Douching or using vaginal spermicides or medicines a day or two before a Pap test can also wash away abnormal cells and thus reduce the test's accuracy.
Also, there is a small percentage of women who develop a rare form of aggressive cervical cancer that can develop to an advanced stage in less than a year. In addition, cervical cancer will continue to occur in women who don't receive regular gynecological exams and Pap tests. Most health professionals recommend that all women who are or have been sexually active or have reached age 18 have a Pap smear and gynecological exam as frequently as each year, but at least every three years, depending on their risk factors for cervical cancer.
There may be one exception to this recommendation: Researchers at Louisiana State University, writing in the Nov. 21, 1996, issue of the New England Journal of Medicine, found that the benefits of Pap tests for most women who have had hysterectomies are limited. The Pap test in such women is used to detect abnormal vaginal cells, and the researchers found the tests of little value for this use in women who had had hysterectomies for reasons other than cancer.
Risk Factors
Evidence collected over the past few decades suggests several risk factors for developing cervical cancer. These include having sexual intercourse before age 18, having several sexual partners, or having a sexual partner who previously had a long-term sexual relationship with a woman who had cervical cancer.
Scientists are closely scrutinizing the sexually transmitted human papillomaviruses (HPVs), some of which cause genital warts. Research strongly suggests some types of HPVs (there are more than 60 different types) can trigger the growth of abnormal cells in the cervix and are likely to play a key role in the development of cervical cancer. Women who have HPV or whose partners have HPV have an increased risk of developing cervical cancer.
However, many women infected with HPV do not develop cervical cancer, and not all women with cervical cancer harbor HPV. This suggests other factors act with HPV to cause cervical cancer. The genital herpes virus may play a role, as may the strength of a woman's immune system. Women infected with HIV, the virus that causes AIDS, are more likely to develop cervical cancer, as are female organ transplant patients who receive drugs that suppress the immune system to prevent rejection of the new organ.
Hormones may also influence the development of cervical cancer. The labeling of oral contraceptives states that some studies have found an increased incidence of cervical cancer in women taking birth control pills, but that this may be related to factors other than the pill. Women whose mothers took the estrogen-like drug diethylstilbestrol (DES) during pregnancy to prevent miscarriage are also more likely to develop cervical cancer. (DES was used to prevent miscarriages from about 1940 to 1970.)
Smoking also elevates the risk of cervical cancer, which rises with the number of cigarettes a woman smokes each day and with the number of years she has smoked. Women exposed to other people's tobacco smoke are also more likely to develop cervical cancer.
Research suggests women can reduce their risk of cervical cancer by using barrier methods of contraception, such as the diaphragm with spermicide and condoms, probably because such methods decrease the risk of being infected by a sexually transmitted disease.
At present, early detection of precancerous tissue remains the most effective way of preventing cervical cancer. When detected in its early stages nearly all cervical cancers can be cured with minor surgery or other practices.
In contrast, fewer than 20 percent of women with advanced cervical cancer survive more than five years, even with treatment, according to the National Cancer Institute. There are nearly 5,000 deaths due to cervical cancer each year in this country. Recent data from NCI reveal that the number of cases of cervical cancer in white women under the age of 50 in the United States has been increasing 3 percent each year since 1986. In contrast, incidence rates are declining in black women of all ages and in white women over age 50. According to the World Health Organization, cervical cancer is also the most common cancer among women in developing countries. New technologies available now may enhance the value of the Pap test, but for women the most important step is getting screened.
In what is thought to be one of the first steps in the development of cervical cancer, the nuclei of cervical cells enlarge and darken. A patch of these abnormal cells is termed a squamous intraepithelial lesion (SIL) because the abnormal cells are present only in the squamous epithelial cells which line the surface of the cervix. SILs are further classified as low-grade if the abnormal cells are of normal size, or high-grade if the cells are smaller than normal.
Low-grade SILs are common; most spontaneously revert to normal. But because some will progress to high-grade SILs and then to cervical cancer, most doctors ask women with this Pap diagnosis to have Pap tests every four to six months for about two years. After three consecutive Pap tests come back negative, women can return to a routine screening protocol.
If repeated Pap tests show persistent abnormalities, however, a woman's doctor may want to confirm the low-grade SIL diagnosis by further scrutinizing the cervix with other procedures.
Colposcopy is a widely used method to check the cervix for abnormal areas. The doctor applies special stains to the cervix and then uses an instrument much like a microscope (called a colposcope) to detect abnormal cells, which turn a different color than healthy cells.
The doctor also may want to remove a small amount of cervical tissue for examination with a biopsy. It also may be necessary to scrape more tissue from inside the cervical opening. These procedures can be done in the doctor's office under local anesthesia.
If the low-grade SIL diagnosis is confirmed, a doctor may ask the patient to continue to have frequent Pap tests. Alternatively, the doctor may prefer to destroy the abnormal area by freezing it (cryosurgery), burning it (cauterization), or by removing it with a laser or electrosurgical device. Such treatment may cause cramping or other pain, bleeding, or a watery discharge.
High-grade SILs rarely regress spontaneously. Most progress to cervical cancer over a period of 10 to 15 years, according to the National Cancer Institute. Women who have high-grade SIL Pap reports usually are asked to undergo a colposcopy or biopsy procedure to confirm diagnosis. Once the high-grade SIL diagnosis is certain, doctors usually destroy the lesion with one of the procedures described in the previous paragraph. Or, the lesion and adjacent tissue may be surgically removed.
If a high-grade SIL progresses to the point that the cell nuclei become jagged or irregular in shape, extremely dark, and enlarged, and the cells themselves are strangely shaped (tadpole- or spindle-shaped, for example, instead of round), the lesion is considered cancerous. If the cancer is limited in scope, it may be treated with some of the same methods used to destroy precancerous lesions. For more widespread cancers, more involved surgery is usually done, removing a larger portion of the cervix or the entire uterus, ovaries or fallopian tubes. Depending on the size and location of the tumor, radiation therapy or chemotherapy may also be necessary.
Publication No. (FDA) 97-4264