Pharmaceutical and Medicine Manufacturing
The pharmaceutical and medicine manufacturing industry has produced a variety of medicinal and other health-related products undreamed of by even the most imaginative apothecaries of the past. These drugs save the lives of millions of people from various diseases and permit many ill people to recover to lead normal lives. Goods and services. Thousands of medications are available today for diagnostic, preventive, and therapeutic uses. In addition to aiding in the treatment of infectious diseases such as pneumonia, tuberculosis, malaria, influenza, and sexually transmitted diseases, these medicines also help prevent and treat cardiovascular disease, asthma, diabetes, hepatitis, cystic fibrosis, and cancer. For example, antinausea drugs help cancer patients endure chemotherapy; clot-buster drugs help stroke patients avoid brain damage; and psychoactive drugs reduce the severity of mental illness for many people. Antibiotics and vaccines have virtually wiped out such diseases as diphtheria, syphilis, and whooping cough. Discoveries in veterinary drugs have controlled various diseases, some of which are transmissible to humans. The U.S. pharmaceutical industry has achieved worldwide prominence through research and development (R&D) on new drugs, and spends a relatively high proportion of its funds on R&D compared with other industries. Each year, pharmaceutical industry testing involves tens of thousands of new substances, yet may eventually yield fewer than 100 new prescription medicines. For the majority of firms in this industry, the actual manufacture of drugs is the last stage in a lengthy process that begins with scientific research to discover new products and to improve or modify existing ones. The R&D departments in pharmaceutical and medicine manufacturing firms start this process by seeking and rapidly testing libraries of thousands to millions of new chemical compounds with the potential to prevent, combat, or alleviate symptoms of diseases or other health problems. Scientists use sophisticated techniques, including computer simulation, combinatorial chemistry, and high-through-put screening (HTS), to hasten and simplify the discovery of potentially useful new compounds. Most firms devote a substantial portion of their R&D budgets to applied research, using scientific knowledge to develop a drug targeted to a specific use. For example, an R&D unit may focus on developing a compound that will effectively slow the advance of breast cancer. If the discovery phase yields promising compounds, technical teams then attempt to develop a safe and effective product based on the discoveries. To test new products in development, a research method called “screening” is used. To screen an antibiotic, for example, a sample is first placed in a bacterial culture. If the antibiotic is effective, it is next tested on infected laboratory animals. Laboratory animals also are used to study the safety and efficacy of the new drug. A new drug is selected for testing on humans only if it promises to have therapeutic advantages over drugs already in use, or is safer. Drug screening is an incredibly risky, laborious, and costly process—only 1 in every 5,000 to 10,000 compounds screened eventually becomes an approved drug. After laboratory screening, firms conduct clinical investigations, or “trials,” of the drug on human patients. Human clinical trials normally take place in three phases. First, medical scientists administer the drug to a small group of healthy volunteers to determine and adjust dosage levels, and monitor for side effects. If a drug appears useful and safe, additional tests are conducted in two more phases, each phase using a successively larger group of volunteers or carefully selected patients, sometimes upwards of 10,000 individuals. After a drug successfully passes animal and clinical tests, the U.S. Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) must review the drug’s performance on human patients before approving the substance for commercial use. The entire process, from the first discovery of a promising new compound to FDA approval, can take over a decade and cost hundreds of millions of dollars. After FDA approval, problems of production methods and costs must be worked out before manufacturing begins. If the original laboratory process of preparing and compounding the ingredients is complex and too expensive, pharmacists, chemists, chemical engineers, packaging engineers, and production specialists are assigned to develop a manufacturing process economically adaptable to mass production. After the drug is marketed, new production methods may be developed to incorporate new technology or to transfer the manufacturing operation to a new production site. In many production plants, pharmaceutical manufacturers have developed a high degree of automation. Milling and micronizing machines, which pulverize substances into extremely fine particles, are used to reduce bulk chemicals to the required size. These finished chemicals are combined and processed further in mixing machines. The mixed ingredients may then be mechanically capsulated, pressed into tablets, or made into solutions. One type of machine, for example, automatically fills, seals, and stamps capsules. Other machines fill bottles with capsules, tablets, or liquids, and seal, label, and package the bottles. Quality control and quality assurance are vital in this industry. Many production workers are assigned full time to quality control and quality assurance functions, whereas other employees may devote part of their time to these functions. For example, although pharmaceutical company sales representatives, often called detailers, work primarily in marketing, they engage in quality control when they assist pharmacists in checking for outdated products. Industry organization. The pharmaceutical and medicine manufacturing industry consists of over 2,500 places of employment, located throughout the country. These include establishments that make pharmaceutical preparations or finished drugs; biological products, such as serums and vaccines; bulk chemicals and botanicals used in making finished drugs; and diagnostic substances such as pregnancy and blood glucose kits. Recent developments. Advances in biotechnology are transforming drug discovery and development. Bioinformatics, a branch of biotechnology using information technologies to work with biological data like DNA, is a particularly dynamic new area of work. Scientists have learned a great deal about human genes, but the real work—translating that knowledge into viable new drugs—has only recently begun. So far, millions of people have benefited from medicines and vaccines developed through biotechnology, and several hundred new biotechnologically-derived medicines are currently in the pipeline. These new medicines, all of which are in human clinical trials or awaiting FDA approval, include drugs for cancer, infectious diseases, autoimmune diseases, neurologic disorders, and HIV/AIDS and related conditions. Many new drugs are expected to be developed in the coming years. Advances in technology and the knowledge of how cells work will allow pharmaceutical and medicine manufacturing makers to become more efficient in the drug discovery process. New technology allows life scientists to test millions of drug candidates far more rapidly than in the past. Other new technology, such as regenerative therapy using stem cell research, also will allow the natural healing process to work faster, or enable the regrowth of missing or damaged tissue. There is a direct relationship between gene discovery and identification of new drugs—the more genes identified the more paths available for drug discovery. Data obtained from the mapping of the human genome can be compared with known gene sequences to identify the proteins produced by each gene, and the effect of those proteins on the body. The study of these gene sequences and the varieties of proteins they produce contribute to the development of new medicines, both biotechnological and chemical. Among other uses, new genetic technology is being explored to develop vaccines to prevent or treat diseases that have eluded traditional vaccines, such as AIDS, malaria, tuberculosis, and cervical cancer.
Hours. In 2006 production workers in pharmaceutical and medicine manufacturing worked an average of 41.8 hours per week, compared with 33.9 for workers in all industries. Some employees work in plants that operate around the clock—three shifts a day, 7 days a week. In most plants, workers receive extra pay when assigned to the second or third shift. Because drug production is subject to little seasonal variation or fluctuation in economic activity, work is steady. Work environment. Working conditions in pharmaceutical plants are better than those in most other manufacturing plants. Much emphasis is placed on keeping equipment and work areas clean because of the danger of contamination. Plants usually are air-conditioned, well lighted, and quiet. Ventilation systems protect workers from dust, fumes, and disagreeable odors. Special precautions are taken to protect the relatively small number of employees who work with infectious cultures and poisonous chemicals. With the exception of work performed by material handlers and maintenance workers, most jobs require little physical effort. In 2006, the incidence of work-related injury and illness was 2.4 cases per 100 full-time workers, compared with 6.0 per 100 for all manufacturing industries and 4.4 per 100 for the entire private sector.
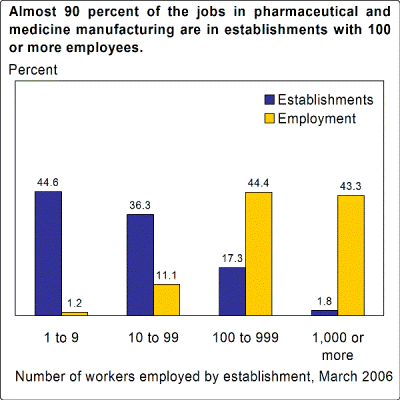
Pharmaceutical and medicine manufacturing provided 292,000 wage and salary jobs in 2006. Pharmaceutical and medicine manufacturing establishments usually employ many workers. Nearly 90 percent of this industry’s jobs in 2006 were in establishments that employed more than 100 workers (chart 1). Most jobs are in California, Illinois, Texas, Indiana, New Jersey, New York, North Carolina, and Pennsylvania.
Under the North American Industry Classification System (NAICS), workers in research and development (R&D) establishments that are not part of a manufacturing facility are included in a separate industry—research and development in the physical, engineering, and life sciences. However, due to the importance of R&D work to the pharmaceutical and medicine manufacturing industry, drug-related R&D is discussed in this statement even though a large proportion of pharmaceutical industry-related R&D workers are not included in the employment data.
About 28 percent of all jobs in the pharmaceutical and medicine manufacturing industry are in professional and related occupations, mostly scientists and science technicians. About 16 percent are in management occupations, another 13 percent are in office and administrative support, and 3 percent are in sales and related occupations. About 3 out of 10 jobs in the industry are in production occupations, including both low skilled and high skilled jobs (table 1). Professional and related occupations. Scientists, engineers, and technicians conduct research to develop new drugs. Others work to streamline production methods and improve environmental and quality control. Life scientists are among the largest scientific occupations in this industry. Most of these scientists are biological and medical scientists who produce new drugs using biotechnology to recombine the genetic material of animals or plants. Biological scientists normally specialize in a particular area. Biologists and bacteriologists study the effect of chemical agents on infected animals. Biochemists study the action of drugs on body processes by analyzing the chemical combination and reactions involved in metabolism, reproduction, and heredity. Microbiologists grow strains of microorganisms that produce antibiotics. Physiologists investigate the effect of drugs on body functions and vital processes. Pharmacologists and zoologists study the effects of drugs on animals. Virologists grow viruses, and develop vaccines and test them in animals. Botanists, with their special knowledge of plant life, contribute to the discovery of botanical ingredients for drugs. Other biological scientists include pathologists, who study normal and abnormal cells or tissues, and toxicologists, who are concerned with safety, dosage levels, and the compatibility of different drugs. Medical scientists, who also may be physicians, conduct clinical research, test products, and oversee human clinical trials. The work of physical scientists, particularly chemists, also is important in the development of new drugs. Combinatorial and computational chemists create molecules and test them rapidly for desirable properties. Organic chemists, often using combinatorial chemistry, then combine new compounds for biological testing. Physical chemists separate and identify substances, determine molecular structure, help create new compounds, and improve manufacturing processes. Radiochemists trace the course of drugs through body organs and tissues. Pharmaceutical chemists set standards and specifications for the form of products and for storage conditions; they also see that drug labeling and literature meet the requirements of State and Federal laws. Analytical chemists test raw and intermediate materials and finished products for quality. Science technicians, such as biological and chemical technicians, play an important part in research and development of new medicines. They set up, operate, and maintain laboratory equipment, monitor experiments, analyze data, and record and interpret results. Science technicians usually work under the supervision of scientists or engineers. Although engineers account for a small fraction of scientific and technical workers, they make significant contributions toward improving quality control and production efficiency. Chemical engineers design equipment and devise manufacturing processes. Bioprocess engineers, who are similar to chemical engineers, design fermentation vats and various bioreactors for microorganisms that will produce a given product. Industrial engineers plan equipment layout and workflow to maintain efficient use of plant facilities. Management, business, and financial occupations. At the top of the managerial group are executives who make policy decisions concerning matters of finance, marketing, and research. Other managerial workers include natural sciences managers and industrial production managers. Other occupations. Workers in office and administrative support occupations include secretaries and administrative assistants, general office clerks, and others who keep records on personnel, payroll, raw materials, sales, and shipments. Sales representatives, wholesale and manufacturing, describe their company’s products to physicians, pharmacists, dentists, and health services administrators. These workers serve as lines of communication between their companies and clients. Most plant workers fall into one of two occupational groups: Production workers who operate drug-producing equipment, inspect products, and install, maintain, and repair production equipment; and transportation and material moving workers who package and transport the drugs. Workers among the larger of the production occupations, assemblers and fabricators, perform all of the assembly tasks assigned to their teams, rotating through the different tasks rather than specializing in a single task. They also may decide how the work is to be assigned and how different tasks are to be performed. Other production workers specialize in one part of the production process. Chemical processing machine setters, operators, and tenders, such as pharmaceutical operators, control machines that produce tablets, capsules, ointments, and medical solutions. Included among these operators are mixing and blending machine setters, operators, and tenders, who tend milling and grinding machines that reduce mixtures to particles of designated sizes. Extruding, forming, pressing, and compacting machine setters, operators, and tenders tend tanks and kettles in which solutions are mixed and compounded to make up creams, ointments, liquid medications, and powders. Crushing, grinding, polishing, mixing, and blending workers operate machines that compress ingredients into tablets. Coating, painting, and spraying machine setters, operators, and tenders, often called capsule coaters, control a battery of machines that apply coatings that flavor, color, preserve, or add medication to tablets, or control disintegration time. Throughout the production process, inspectors, testers, sorters, samplers, and weighers ensure consistency and quality. For example, ampoule examiners inspect ampoules for discoloration, foreign particles, and flaws in the glass. Tablet testers inspect tablets for hardness, chipping, and weight to assure conformity with specifications. After the drug is prepared and inspected, it is bottled or otherwise packaged by packaging and filling machine operators and tenders. Plant workers who do not operate or maintain equipment perform a variety of other tasks. Some drive industrial trucks or tractors to move materials around the plant, load and unload trucks and railroad cars, or package products and materials by hand.
Training requirements for jobs in the pharmaceutical and medicine manufacturing industry range from a few hours of on-the-job training to years of formal education plus job experience. More than 6 out of 10 of all workers have a bachelor’s, master’s, professional, or Ph.D. degree—more than twice the proportion for all industries combined. Production occupations. Manufacturers usually hire inexperienced workers and train them on the job; high school graduates generally are preferred. Beginners in production jobs assist experienced workers and learn to operate processing equipment. With experience, employees may advance to more skilled jobs in their departments. The industry places a heavy emphasis on continuing education for employees, and many firms provide classroom training in safety, environmental and quality control, and technological advances. Many companies encourage production workers to take courses related to their jobs at local schools and technical institutes or to enroll in correspondence courses. College courses in chemistry and related areas are particularly encouraged for highly skilled production workers who operate sophisticated equipment. Some companies reimburse workers for part, or all, of their tuition. Skilled production workers with leadership ability may advance to supervisory positions. Science technician occupations. To fill these jobs, most companies prefer to hire graduates of technical institutes or community colleges or those who have completed college courses in chemistry, biology, mathematics, or engineering. Some companies, however, require science technicians to hold a bachelor’s degree in a biological or chemical science. In many firms, newly hired workers begin as laboratory helpers or aides, performing routine jobs such as cleaning and arranging bottles, test tubes, and other equipment. The experience required for higher level technician jobs varies from company to company. Usually, employees advance over a number of years from assistant technician, to technician, to senior technician, and then to technical associate, or supervisory technician. Scientific and engineering occupations. A Bachelor of Science degree is typically the minimum requirement for these workers. Scientists involved in research and development usually have a master’s or doctoral degree. A doctoral degree is generally the minimum requirement for medical scientists, and those who administer drug or gene therapy to patients in clinical trials must have a medical degree. Because biotechnology is not one discipline, but the interaction of several disciplines, the best preparation for work in biotechnology is training in a traditional biological science, such as genetics, molecular biology, biochemistry, virology, or biochemical engineering. Individuals with a scientific background and several years of industry experience may eventually advance to managerial positions. Some companies offer training programs to help scientists and engineers keep abreast of new developments in their fields and to develop administrative skills. These programs may include meetings and seminars with consultants from various fields. Many companies encourage scientists and engineers to further their education; some companies provide financial assistance or full reimbursement of expenses for this purpose. Publication of scientific papers also is encouraged. Sales and related occupations. Pharmaceutical manufacturing companies prefer to hire college graduates, particularly those with strong scientific backgrounds. In addition to a 4-year degree, most newly employed pharmaceutical sales representatives complete rigorous formal training programs revolving around their company’s product lines.
Employment is expected to increase through 2016. Pharmaceutical and medicine manufacturing will be one of the fastest growing manufacturing industries. Employment change. The number of wage and salary jobs in pharmaceutical and medicine manufacturing is expected to increase by 24 percent over the 2006-16 period, compared with the 11 percent projected for all industries combined. Pharmaceutical and medicine manufacturing ranks among the fastest growing manufacturing industries. Demand for this industry’s products is expected to remain strong. Even during fluctuating economic conditions, there will be a market for over-the-counter and prescription drugs, including the diagnostics used in hospitals, laboratories, and homes; the vaccines used routinely on infants and children; analgesics and other symptom-easing drugs; antibiotics and other drugs for life-threatening diseases; and “lifestyle” drugs for the treatment of nonlife-threatening conditions. The use of drugs, particularly antibiotics and vaccines, has helped to eradicate or limit a number of deadly diseases, but many others, such as cancer, Alzheimer’s, and heart disease, continue to elude cures. Ongoing research and the manufacture of new products to combat these diseases will continue to contribute to employment growth. Demand also is expected to increase as the population expands because so many of the pharmaceutical and medicine manufacturing industry’s products are related to preventive or routine healthcare, rather than just illness. The growing number of older people, who will require more healthcare services, will further stimulate demand—along with the growth of both public and private health insurance programs, which increasingly cover the cost of drugs and medicines. Another factor propelling demand is the increasing popularity of “lifestyle” drugs. These drugs treat symptoms of chronic nonlife-threatening conditions resulting from aging or genetic predisposition and can enhance one’s self-confidence or physical appearance. Other factors expected to increase the demand for drugs include greater personal income and the rising health consciousness and expectations of the general public. Despite the increasing demand for drugs, drug producers and buyers are expected to place more emphasis on cost effectiveness, due to concerns about the cost of health care, including prescription drugs. Growing competition from the producers of generic drugs also may exert cost pressures on many firms in this industry, particularly as brand-name drug patents expire. In addition, the average time for the FDA to review “nonpriority” drug applications is becoming longer, further delaying the time a drug comes to market. These factors, combined with continuing improvements in manufacturing processes, are expected to result in rapid employment growth over the 2006-16 period, but nevertheless slower than occurred during the previous 10-year period. Strong demand is anticipated for professional occupations—especially for life and physical scientists engaged in R&D, the backbone of the pharmaceutical and medicine manufacturing industry. Much of the basic biological research done in recent years has resulted in new knowledge, including the successful identification of genes. Life and physical scientists will be needed to take this knowledge to the next stage, which is to understand how certain genes function so that gene therapies can be developed to treat diseases. Computer specialists such as systems analysts, biostatisticians, and computer support specialists also will be in demand as disciplines such as biology, chemistry, and electronics continue to converge and become more interdisciplinary, creating demand in rapidly emerging fields such as bioinformatics and nanotechnology. Strong demand also is projected for production occupations. Employment of office and administrative support workers is expected to grow more slowly than the industry as a whole, as companies streamline operations and increasingly rely on computers Job prospects. Job opportunities in most occupations should be good, particularly for those employees with science and engineering backgrounds. Unlike many other manufacturing industries, the pharmaceutical and medicine manufacturing industry is not highly sensitive to changes in economic conditions. Even during periods of high unemployment, work is likely to be relatively stable in this industry. Additional openings will arise from the need to replace workers who transfer to other industries, retire, or leave the workforce for other reasons.
Industry earnings. Earnings of workers in the pharmaceutical and medicine manufacturing industry are higher than the average for all manufacturing industries. In 2006, production or nonsupervisory workers in this industry averaged $891 a week, while those in all manufacturing industries averaged $695 a week. Earnings in selected occupations in pharmaceutical and medicine manufacturing appear in table 2.
Benefits and union membership. Workers in the pharmaceutical and medicine manufacturing industry generally receive paid sick and vacation leave and health insurance, and many employers contribute to pension plans and life insurance. Some firms may offer their medicines to employees at a reduced cost. Only about 5 percent of the workers in the pharmaceutical and medicine manufacturing industry are union members or are covered by a union contract, compared with about 13 percent of workers throughout private industry.
For additional information about careers in pharmaceutical and medicine manufacturing, write to the human resources departments of individual pharmaceutical and medicine manufacturing companies. For information about careers in biotechnology, contact:
For information on careers in pharmaceutical and medicine manufacturing, contact:
Information on these key pharmaceutical and medicine manufacturing occupations may be found in the 2008-09 edition of the Occupational Outlook Handbook.
3254
Suggested citation: Bureau of Labor Statistics, U.S. Department of Labor, Career Guide to Industries, 2008-09 Edition, Pharmaceutical and Medicine Manufacturing, on the Internet at http://www.bls.gov/oco/cg/cgs009.htm
(visited
September 17, 2008
).
Last Modified Date: December 18, 2007 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tools |
Calculators |
Help |
Info |