Research Highlights
June 2007
Researchers Develop, Improve and Enhance Technologies for Rapid Analysis of Complex Samples
FAIMS/IMS is meeting the proteomics throughput challenge
Results: Scientists at Pacific Northwest National Laboratory can now analyze complex biochemical and other samples in minutes, opening doors to scientific study and practical applications in areas that were hamstrung by long waits for results. By coupling FAIMS (Field Asymmetric waveform Ion Mobility Spectrometry) and conventional Ion Mobility Spectrometry (IMS), they've developed a broadly useful new technology capable of quickly separating, identifying and quantifying the components of complex mixtures. Analyses can potentially be done in seconds to minutes rather than hours to tens of hours.
Why it matters: The speed and separation power of FAIMS/IMS (in conjunction with mass spectrometry, MS) create a new realm of research opportunities in areas such as proteomics, metabolomics and characterization of natural fuels and organic matter in soil. The technology could also help determine the health risks of low-level radiation exposure, understand natural bioremediation processes to enable their control and detect explosives and chemical or biological warfare agents.
Technologies for separating and characterizing ions based on their mobility in gases have been around for more than three decades. Conventional IMS that distinguishes ions by absolute mobility (that depends on the collision cross sections of ions with molecules of a buffer gas, such as air or nitrogen) has been used since 1970. The more recent FAIMS technique separates ions by the difference between their mobility in strong and weak electric fields.
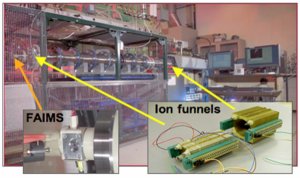
Ultra-high sensitivity Field Asymmetric waveform Ion Mobility Spectrometry (FAIMS/IMS) provides an effective separation power equivalent to liquid chromatography, but 100-fold faster. The combination was greatly advanced using PNNL ion funnel technology. Enlarged View
Advantages of the FAIMS/IMS combination are speed (analyses require seconds to minutes instead of hours to tens of hours for condensed-phase alternatives such as liquid chromatographic methods at comparable separation power) and capability to characterize ion structures. Coupling IMS and FAIMS to electrospray ionization mass spectrometry (ESI-MS) has greatly expanded their utility, enabling new applications in biomedical and nanomaterials research by allowing analyses of more complex mixtures.
"FAIMS is the first but likely not the only example of a nonlinear IMS method. Combining various nonlinear and linear IMS phenomena will allow further novel approaches to analytical separations," notes Alex Shvartsburg, one of the lead scientists for the FAIMS/IMS.
Methods: FAIMS/IMS/MS is used at PNNL for high-throughput proteomic analyses and conformational characterization of proteins. The results of FAIMS/IMS development and application have been published in leading scientific journals. In particular, PNNL scientists have
- Designed and constructed the most sensitive ESI/IMS/time-of-flight (TOF) MS instrument to date by using electrodynamic ion funnels at both ends of the IMS drift tube for effective ion focusing and accumulation at the ESI/IMS interface and near-perfect ion transmission from IMS to TOF MS.
- Developed the first ESI/FAIMS/IMS/TOF MS platform and demonstrated its utility for high-throughput analyses of complex mixtures and characterization of macromolecular conformations and protein folding.
- Established a comprehensive FAIMS modeling capability and used it to guide the development of advanced FAIMS technology, including a new planar FAIMS design achieving record resolving power, the higher-order differential IMS (HODIMS) concept, and a slit-aperture interface for efficient coupling of planar FAIMS to MS and IMS/MS stages.
Acknowledgments: The research team includes Keqi Tang, Alex Shvartsburg, David Prior, Mikhail Belov, Erin Baker, Brian Clowers, and Dick Smith, all at PNNL. Portions of this work were supported by PNNL Biomolecular Systems Initiative, and the National Institutes of Health National Center for Research Resources. The work was done at the W.R. Wiley Environmental Molecular Sciences Laboratory, a U.S. Department of Energy user facility at PNNL.
References:
Shvartsburg AA, F Li, K Tang, and RD Smith. 2007. "Distortion of ion structures by field asymmetric waveform ion mobility spectrometry." Anal. Chem. 79(4):1523.
Belov ME, MA Buschbach, DC Prior, K Tang, and RD Smith. 2007. "Multiplexed ion mobility spectrometry-orthogonal time-of-flight mass spectrometry." Anal. Chem. 79(6):2451.
Shvartsburg AA, F Li, K Tang, and RD Smith. 2006. "Characterizing the structures and folding of free proteins using 2-D gas-phase separations: observation of multiple unfolded conformers." Anal. Chem. 78(10):3304.
Shvartsburg AA, F Li, K Tang, and RD Smith. 2006. "High-resolution field asymmetric waveform ion mobility spectrometry using new planar geometry analyzers." Anal. Chem. 78(11):3706.
Shvartsburg AA, T Bryskiewicz, R Purves, K Tang, R Guevremont, and RD Smith. 2006. "Field asymmetric waveform ion mobility spectrometry studies of proteins: dipole alignment in ion mobility spectrometry?" J. Phys. Chem. B 110(43):21966.
Shvartsburg AA, SV Mashkevich, and RD Smith. 2006. "Feasibility of higher-order differential ion mobility separations using new asymmetric waveforms." J.Phys. Chem. A 110(8):2663.
Tang K, F Li, AA Shvartsburg, EF Strittmatter, and RD Smith. 2005. "Two-dimensional gas-phase separations coupled to mass spectrometry for analysis of complex mixtures." Anal. Chem. 77(19):6381.
Tang K, AA Shvartsburg, H Lee, DC Prior, MA Buschbach, F Li, AV Tolmachev, GA Anderson, and RD Smith. 2005. "High-Sensitivity Ion Mobility Spectrometry/Mass Spectrometry Using Electrodynamic Ion Funnel Interfaces." Anal. Chem. 77(10):3330.



